1 Dec. 6, 2017 Procedure Code List for Preauthorization for Blue Cross
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Procedure Code List Effective Jan. 1, 2020 for Preauthorization for Blue Cross and Blue Shield of New Mexico Medicare Advantage Members Only
Procedure Code List Effective Jan. 1, 2020 for Preauthorization for Blue Cross and Blue Shield of New Mexico Medicare Advantage Members only Beginning Jan. 1, 2020, providers will be required to obtain preauthorization through Blue Cross and Blue Shield of New Mexico (BCBSNM), Optum, or eviCore for certain procedures for Blue Cross Medicare Advantage members as noted in the MAPD Benefit Preauthorization Procedure Code List, Effective 1/1/2020, below. For members NOT attributed to Optum, preauthorization should be obtained from BCBSNM unless the applicable entry in the MAPD Benefit Preauthorization Procedure Code List references eviCore. For members attributed to Optum, preauthorization should be obtained from Optum, even if the applicable entry in the MAPD Benefit Preauthorization Procedure Code List references eviCore. Any entry that references eviCore should be preauthorized through eviCore except for members attributed to Optum. The member's ID Card will indicate that the member is attributed to Optum. Services performed without benefit preauthorization may be denied for payment in whole or in part, and you may not seek reimbursement from members. Member eligibility and benefits should be checked prior to every scheduled appointment. Eligibility and benefit quotes include membership status, coverage status and other important information, such as applicable copayment, coinsurance and deductible amounts. It is strongly recommended that providers ask to see the member's ID card for current information and a photo ID to guard against medical identity theft. A referral to an out-of-plan or out-of-network provider which is necessary due to network inadequacy or continuity of care must be reviewed by the BCBSNM Utilization Management or DMG (if the member is attributed to DMG this information will be reflected on the ID card) prior to a BCBSNM patient receiving care. -

Reframing Psychiatry for Precision Medicine
Reframing Psychiatry for Precision Medicine Elizabeth B Torres 1,2,3* 1 Rutgers University Department of Psychology; [email protected] 2 Rutgers University Center for Cognitive Science (RUCCS) 3 Rutgers University Computer Science, Center for Biomedicine Imaging and Modelling (CBIM) * Correspondence: [email protected]; Tel.: (011) +858-445-8909 (E.B.T) Supplementary Material Sample Psychological criteria that sidelines sensory motor issues in autism: The ADOS-2 manual [1, 2], under the “Guidelines for Selecting a Module” section states (emphasis added): “Note that the ADOS-2 was developed for and standardized using populations of children and adults without significant sensory and motor impairments. Standardized use of any ADOS-2 module presumes that the individual can walk independently and is free of visual or hearing impairments that could potentially interfere with use of the materials or participation in specific tasks.” Sample Psychiatric criteria from the DSM-5 [3] that does not include sensory-motor issues: A. Persistent deficits in social communication and social interaction across multiple contexts, as manifested by the following, currently or by history (examples are illustrative, not exhaustive, see text): 1. Deficits in social-emotional reciprocity, ranging, for example, from abnormal social approach and failure of normal back-and-forth conversation; to reduced sharing of interests, emotions, or affect; to failure to initiate or respond to social interactions. 2. Deficits in nonverbal communicative behaviors used for social interaction, ranging, for example, from poorly integrated verbal and nonverbal communication; to abnormalities in eye contact and body language or deficits in understanding and use of gestures; to a total lack of facial expressions and nonverbal communication. -

Sensory Deficits in a Mouse Model of Christianson Syndrome Tarheen
Sensory Deficits in a Mouse Model of Christianson Syndrome Tarheen Fatima Department of Physiology McGill University, Montreal April 2019 A thesis submitted to McGill University in partial fulfillment of the requirements of the degree of Master of Science. © Tarheen Fatima 2019 Abstract Christianson Syndrome (CS) is a recently characterized X-linked neurodevelopmental disorder caused by loss-of-function mutations in the gene slc9a6, encoding the endosomal Na+/H+ Exchanger 6 (NHE6). The disorder is associated with developmental delay, intellectual disability, loss of motor coordination, mutism, ataxia, epilepsy, as well as autistic features. In addition to these symptoms, CS patients exhibit elevated pain thresholds to noxious stimuli as well as discomfort at normally innocuous stimuli. The underlying causes of these sensory deficits are yet to be determined. Hence, this study aims at understanding how loss-of-function of NHE6 affects transmission and processing of pain. To this end, we examined the expression of NHE6 in peripheral and central neurons implicated in sensation and interpretation of pain. Additionally, we characterized the nociceptive behaviour of NHE6 knockout (KO) mice using a battery of behaviour tests. Our immunohistochemical experiments demonstrate that NHE6 is highly expressed in nociceptive, small-diameter dorsal root ganglia (DRG) neurons. Moreover, mice lacking NHE6 display decreased responses to noxious mechanical, thermal and chemical stimuli but are more responsive to noxious cold than wild-type littermates. Interestingly, immunohistochemical characterization of DRG tissue from aged NHE6 null mice indicates a decrease in some neuronal subsets suggesting cell death. Finally, using light brush-induced Fos activation in the dorsal horn, we found that the spinal processing of innocuous stimuli is not different between wildtype and NHE6 knockout mice. -

PT/OT Therapy Intake Form Required for All MSK Conditions (Including Hand) Please Use This Fax Form for NON-URGENT Requests Only
Musculoskeletal Program: PT/OT Therapy Intake Form Required for all MSK Conditions (Including Hand) Please use this fax form for NON-URGENT requests only. Failure to provide all relevant information may delay the determination. Phone and fax numbers may be found on eviCore.com under the Guidelines and Forms section. You may also log into the provider portal located on the site to submit an authorization request. URGENT (same day) REQUESTS MUST BE SUBMITTED BY PHONE Previous Reference/Auth Number (If Continued Care): Date of Submission: Place of Service: ****** Note: This is REQUIRED for WellCare Submissions ******* Service Type Requested: Physical Therapy Occupational Therapy First Name: MI: Last Name: Member ID: DOB (mm/dd/yyyy): Gender: Male Female Street Address: Apt #: City: State: Zip: PATIENT Home Phone: Cell Phone: Primary: Home Cell Member Health Plan/Insurer: First Name: Last Name: Primary Specialty: TIN: NPI: Physician Phone: Physician Fax: Address: Suite #: City: State: Zip: PROVIDER Office Contact: Ext: Email: Diagnoses: Code Description Code Description Start Date for this Request: This is a (select the most appropriate): New condition not previously treated Same/previous condition Date of initial evaluation: Date of onset of condition: Date of current findings: Primary Treatment Area: Spine: Cervical / Upper Thoracic Lower Thoracic / Lumbar / Pelvis Upper Extremity: Shoulder / Arm Elbow / Wrist / Forearm Hand Lower Extremity: Hip / Thigh Knee Ankle / Foot Other: Pelvic Pain / Incontinence Secondary Treatment Area: Spine: -
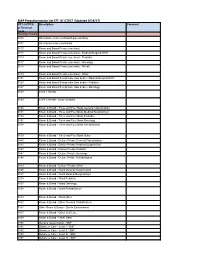
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17)
MAP Preauthorization List EFF: 8/1/2017 (Updated 8/24/17) CPT, HCPCS Description Comment or Revenue Code Revenue Codes 0100 All inclusive room and board plus ancillary 0101 All inclusive room and board 0110 Room and Board Private (one bed) 0111 Room and Board Private (one bed) - Medical/Surgical/GYN 0113 Room and Board Private (one bed) - Pediatric 0117 Room and Board Private (one bed) - Oncology 0118 Room and Board Private (one bed) - Rehab 0119 Room and Board Private (one bed) - Other 0121 Room and Board Semiprivate (two beds) - Medical/Surgical/GYN 0123 Room and Board Semiprivate (two beds) - Pediatric 0127 Room and Board Semiprivate (two beds) - Oncology 0128 Level 1 Rehab 0129 Level 2 Rehab - acute complex 0130 Room & Board - Three and Four Beds General Classification 0131 Room & Board - Three and Four Beds Medical/Surgical/Gyn 0133 Room & Board - Three and Four Beds Pediatric 0137 Room & Board - Three and Four Beds Oncology 0138 Room & Board - Three and Four Beds Rehabilitation 0139 Room & Board - Three and Four Beds Other 0140 Room & Board - Deluxe Private General Classification 0141 Room & Board - Deluxe Private Medical/Surgical/Gyn 0143 Room & Board - Deluxe Private Pediatric 0147 Room & Board - Deluxe Private Oncology 0148 Room & Board - Deluxe Private Rehabilitation 0149 Room & Board - Deluxe Private Other 0150 Room & Board - Ward General Classification 0151 Room & Board - Ward Medical/Surgical/Gyn 0153 Room & Board - Ward Pediatric 0157 Room & Board - Ward Oncology 0158 Room & Board - Ward Rehabilitation 0159 Room & Board - -

What Is Christianson Syndrome? by Dr
What is Christianson Syndrome? By Dr. Eric Morrow Christianson syndrome is a genetic disorder that affects brain development. It is an “X-linked” disorder (see “Why is CS found only in boys?” section for a description of what X-linked means). Symptoms of CS usually appear in infancy and include epilepsy and intellectual disability. Although the exact prevalence of Christianson syndrome is unknown, a genetic study by Tarpey and colleagues identified Christianson Syndrome in 2 of about 200 families (about 1% of families with apparent X-linked developmental disabilities). This was among the most common genetic change in X-linked forms of intellectual disability. What are the causes of Christianson syndrome? Christianson syndrome is caused by changes in the gene, SLC9A6. Genes are important because they code for proteins, which carry out essential processes in the cells of our bodies. The SLC9A6 gene codes for the NHE6 protein. NHE6 is part of a family of proteins that are sodium (Na+)/hydrogen (H+) exchangers. The NHE6 protein is found in endosomes, which take up molecules to be destroyed or recycled for other processes in our body. NHE6 makes sure that these endosomes have the correct intra-endosomal acid level by letting in Na into the endosome and getting rid of hydrogen, which is the acid molecule. The acid level regulates protein turnover. My analogy our stomach acid, the acidity in endosomes helps degrade cellular proteins. These genetic changes in SLC9A6 cause the NHE6 protein to not work properly. You may have heard words such as “loss-of-function”, “truncation” or “frame- shift”, which describe how the NHE6 protein cannot be made in the cells to carry out these processes in the endosome. -

114.3 Cmr: Division of Health Care Finance and Policy Ambulatory Care
114.3 CMR: DIVISION OF HEALTH CARE FINANCE AND POLICY AMBULATORY CARE 114.3 CMR 40.00: RATES FOR SERVICES UNDER M.G.L. c. 152, WORKERS’ COMPENSATION ACT Section 40.01: General Provisions 40.02: General Definitions 40.03: Service and Rate Coverage Provisions 40.04: Provisions Affecting Eligible Providers 40.05: Policies for Individual Service Types 40.06: Fees 40.07: Appendices 40.08: Severability 40.01: General Provisions (1) Scope, Purpose and Effective Date. 114.3 CMR 40.00 governs the payment rates effective April 1, 2009 for purchasers of health care services under M.G.L. c. 152, the Workers’ Compensation Act. Payment rates for services provided by hospitals are set forth in 114.1 CMR 41.00. Program policies relating to medical necessity and clinical appropriateness are determined pursuant to M.G.L. c. 152 and 452 CMR 6.00. (2) Coverage. The payment rates set forth in 114.3 CMR 40.06 are full payment for services provided under M.G.L. c. 152, § 13, including any related administrative or overhead costs. The insurer, employer and health care service provider may agree upon a different payment rate for any service set forth in the fee schedule in 114.3 CMR 40.00. No employee may be held liable for the payment for health care services determined compensable under M.G.L. c. 152, § 13. (3) Administrative Bulletins. The Division may issue administrative bulletins to clarify substantive provisions of 114.3 CMR 40.00, or to publish procedure code updates and corrections. For coding updates and correction, the bulletin will list: (a) new code numbers for existing codes, with the corresponding cross references between existing and new codes numbers; (b) deleted codes for which there are no corresponding new codes; and (c) codes for entirely new services that require pricing. -

Artificial Finger Joint Replacement Due to a Giant Cell Tumor of the Tendon Sheath with Bone Destruction: a Case Report
3502 ONCOLOGY LETTERS 10: 3502-3504, 2015 Artificial finger joint replacement due to a giant cell tumor of the tendon sheath with bone destruction: A case report HUI LU, HUI SHEN, QIANG CHEN, XIANG-QIAN SHEN and SHOU-CHENG WU Department of Hand Surgery, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang 310003, P.R. China Received October 24, 2014; Accepted September 25, 2015 DOI: 10.3892/ol.2015.3813 Abstract. The current study presents the case of a 25-year-old excised completely and the function of the joints was recov- male who developed tumor recurrence of the proximal ered. phalange of the ring finger on the right hand 4 years after partial tumor resection surgery. An X-ray of the right hand Case report showed that the distal bone of the proximal phalange on the ring finger was destroyed. An artificial finger joint replace- A 25-year-old male was admitted to The First Affiliated ment was performed using a silicone joint for this unusual Hospital, College of Meicine, ZheJiang University (Hangzhou, tumor recurrence. The pathological findings were indicative of Zhejiang, China) with tumor recurrence of the proximal a giant cell tumor of the tendon sheath. As a result of surgery, phalange of the ring finger on the right hand 4 years after the patient's proximal interphalangeal point motion recovered partial tumor resection surgery. The patient had no history to the pre-operative level. The pre-operative and post-operative of trauma or infection and was previously healthy. A physical disabilities of the arm, at shoulder and hand and total activity examination revealed swelling of the proximal phalange of the measurement values were 1.67 and 3.33, and 255 and 243˚, finger without clear cause, multiple nodules in the local areas respectively. -

Luca Bartolini, MD 1 8/9/2021 CURRICULUM VITAE LUCA
8/9/2021 CURRICULUM VITAE LUCA BARTOLINI, MD Director, Pediatric Epilepsy Program Hasbro Children’s Hospital Assistant Professor of Pediatrics Assistant Professor of Neurology Assistant Professor of Neurosurgery The Warren Alpert Medical School of Brown University Email: [email protected] EDUCATION: Undergraduate Liceo Classico “Tito Livio”, Padova, Italy Medical School University of Padova School of Medicine, Padova, Italy 09/2000 – 07/2006 Doctor of Medicine (M.D.) POSTGRADUATE TRAINING: Residency Obstetrics and Gynecology University of Parma, Italy 07/2007 – 01/2008 Pediatrics (Pediatric Neurology Track) University of Padova, Italy 04/2008 – 10/2013 Pediatrics Children’s National Medical Center / The George Washington University, Washington DC 07/2012 – 06/2013 Child Neurology Children’s National Medical Center / The George Washington University, Washington DC 07/2013 – 06/2016 Luca Bartolini, MD 1 Fellowship Neuroscience Research Children’s National Medical Center / The George Washington University, Washington DC National Institutes of Health, Bethesda, MD 07/2016 – 06/2017 Epilepsy National Institutes of Health, Bethesda, MD 07/2017 – 06/2018 Clinical Neurophysiology National Institutes of Health, Bethesda, MD 07/2018 – 06/2019 POSTGRADUATE HONORS AND AWARDS: Research Career Symposium Scholarship American Academy of Neurology 02/2015 Pediatric Epilepsy Fellow Pipeline Travel Scholarship Epilepsy Foundation of America 12/2015 Sentinel of Science Award (Top 10% of Researchers contributing to the peer-review of the field of Medicine) -

Amyloid Clearance Defect in Apoe4 Astrocytes Is Reversed by Epigenetic Correction of Endosomal Ph
Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH Hari Prasada and Rajini Raoa,1 aDepartment of Physiology, The Johns Hopkins University School of Medicine, Baltimore, MD 21205 Edited by Reinhard Jahn, Max Planck Institute for Biophysical Chemistry, Goettingen, Germany, and approved June 6, 2018 (received for review January 28, 2018) Endosomes have emerged as a central hub and pathogenic driver ability (7–9). Patients who have CS also show striking age- of Alzheimer’s disease (AD). The earliest brain cytopathology in dependent neurodegeneration, with prominent glial pathology neurodegeneration, occurring decades before amyloid plaques and phosphorylated tau deposits (7). Female carriers have and cognitive decline, is an expansion in the size and number of learning difficulties and behavioral issues, and some present with endosomal compartments. The strongest genetic risk factor for low Mini-Mental State Examination scores suggestive of early sporadic AD is the e4 allele of Apolipoprotein E (ApoE4). Previous cognitive decline (10). Interestingly, NHE6 was among the most studies have shown that ApoE4 potentiates presymptomatic endo- highly down-regulated genes (up to sixfold) in the elderly (70 y) somal dysfunction and defective endocytic clearance of amyloid brain, compared with the adult (40 y) brain (11). These obser- beta (Aβ), although how these two pathways are linked at a cel- vations led us to consider a broader role for NHE6 in neuro- lular and mechanistic level has been unclear. Here, we show that degenerative disorders, including Alzheimer’s disease (AD), a aberrant endosomal acidification in ApoE4 astrocytes traps the major cause of dementia in the elderly. -

MAP Prior Authorization List EFF: 11/01/2017 (Updated 11/7/2017)
MAP Prior Authorization List EFF: 11/01/2017 (Updated 11/7/2017) CPT, HCPCS Description Comment or Revenue Code Inpatient All Inpatient admissions require authorization Revenue Codes 0100 All inclusive room and board plus ancillary 0101 All inclusive room and board 0110 Room and Board Private (one bed) 0111 Room and Board Private (one bed) - Medical/Surgical/GYN 0113 Room and Board Private (one bed) - Pediatric 0117 Room and Board Private (one bed) - Oncology 0119 Room and Board Private (one bed) - Other 0121 Room and Board Semiprivate (two beds) - Medical/Surgical/GYN 0123 Room and Board Semiprivate (two beds) - Pediatric 0127 Room and Board Semiprivate (two beds) - Oncology 0130 Room & Board - Three and Four Beds General Classification 0131 Room & Board - Three and Four Beds Medical/Surgical/Gyn 0133 Room & Board - Three and Four Beds Pediatric 0137 Room & Board - Three and Four Beds Oncology 0139 Room & Board - Three and Four Beds Other 0140 Room & Board - Deluxe Private General Classification 0141 Room & Board - Deluxe Private Medical/Surgical/Gyn 0143 Room & Board - Deluxe Private Pediatric 0147 Room & Board - Deluxe Private Oncology 0149 Room & Board - Deluxe Private Other 0150 Room & Board - Ward General Classification 0151 Room & Board - Ward Medical/Surgical/Gyn 0153 Room & Board - Ward Pediatric 0157 Room & Board - Ward Oncology 0159 Room & Board - Ward Other 0160 Room & Board - Other General Classification 0164 Other Room & Board - Sterile Environment 0167 Room & Board - Other Self Care 0169 Room & Board - Other Other 0190 General -

184591/2021/Estt-Ne Hr
168 184591/2021/ESTT-NE_HR PUSHPAWATI SINGHANIA HOSPITAL & RESEARCH INSTITUTE A MULTISPECIALITY HOSPITAL (formally known as Puspawati Singhania Research Institute for liver,Renal & Digestive Diseases) (w.e.f. 01.04.2020) OPD CHARGES FEES (Rs) SR. NO. CONSULTANT NAME FIRST SUBS. GASTROENTEROLOGY 1PROF.(DR). R.K.TANDON 20001500 2LT. COL. (DR) ARUN KUMAR 12001200 3DR. DINESH SINGHAL 10001000 4DR. MANOJ KUMAR 10001000 5DR. NRIPEN SAIKIA 10001000 6DR. RAJIV BAIJAL 10001000 7DR. RAHUL GUPTA 20002000 8DR. SHUBHAM VATSYA 900900 SURGICAL GASTROENTEROLOGY & LIVER TRANSPLANT 1DR. K.R VASUDEVAN 12001200 2DR. MANOJ GUPTA 10001000 3DR. BHUSHAN P. BHOLA 12001200 NEPHROLOGY 1DR. SANJEEV SAXENA 12001200 2DR. RAVI BANSAL 10001000 3DR. RAJESH GOEL 800800 UROLOGY 1DR. P.P.SINGH 12001200 2DR. JAGDEEP BALYAN 11001100 3DR. A.S.MALHOTRA 11001100 CARDIOLOGIST 1DR. T.S.KLER 10001000 2DR. VINAYAK AGRAWAL 12001200 3DR. AVINASH VERMA 10001000 4DR. S. N. PATHAK 10001000 5DR. ABHINAV AGGARWAL 10001000 CARDIO SURGERY 1DR. BALRAM AIRAN 10001000 2DR. VIJAY MOHAN KOHLI 12501250 3DR GAURAV GUPTA 10001000 NEUROLOGOY 1DR. SHAMSHER DWIVEDEE 20001800 NEURO SURGERY 1DR. SUMIT GOYAL 10001000 ENDOCRINOLOGY 1DR. MONIKA SHARMA 1001000 PULMONOLOGY 1DR. G.C. KHILNANI 15001500 2DR. NEETU JAIN 10001000 G.I SURGERY 1DR. SANJAY CHAUREY 15001500 2DR. RAJEEV KHANNA 12001000 3DR. HARISH KAPILA 15001500 ONCOLOGY (SURGERY) 1DR. ARVIND KUMAR 15001500 2DR. SALEEM NAYAK 15001500 3DR. VIVEK GUPTA 15001500 4DR. VIKRANT SHARMA 15001500 ONCOLOGY 1DR. AMISH VORA 15001500 2DR. AMIT UPADHYAY 15001500 ORTHOPEDICS 1DR. P.P. KOTWAL 15001500 2DR. GAURAV PRAKASH BHARDWAJ 12001200 Page 1 169 184591/2021/ESTT-NE_HR 3DR. U.K.SADHOO 12001200 4DR. G.S.TUCKER 12001200 5DR. ANIL MISHRA 10001000 6DR.