Exploration of Cyanobacterial Flora from North Solaptehshil of Solapur
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Taluka Center Name Contact Person Address Phone No Mobile No
District Taluka Center Name Contact Person Address Phone No Mobile No Mhosba Gate , Karjat Tal Karjat Dist AHMEDNAGAR KARJAT Vijay Computer Education Satish Sapkal 9421557122 9421557122 Ahmednagar 7285, URBAN BANK ROAD, AHMEDNAGAR NAGAR Anukul Computers Sunita Londhe 0241-2341070 9970415929 AHMEDNAGAR 414 001. Satyam Computer Behind Idea Offcie Miri AHMEDNAGAR SHEVGAON Satyam Computers Sandeep Jadhav 9881081075 9270967055 Road (College Road) Shevgaon Behind Khedkar Hospital, Pathardi AHMEDNAGAR PATHARDI Dot com computers Kishor Karad 02428-221101 9850351356 Pincode 414102 Gayatri computer OPP.SBI ,PARNER-SUPA ROAD,AT/POST- 02488-221177 AHMEDNAGAR PARNER Indrajit Deshmukh 9404042045 institute PARNER,TAL-PARNER, DIST-AHMEDNAGR /221277/9922007702 Shop no.8, Orange corner, college road AHMEDNAGAR SANGAMNER Dhananjay computer Swapnil Waghchaure Sangamner, Dist- 02425-220704 9850528920 Ahmednagar. Pin- 422605 Near S.T. Stand,4,First Floor Nagarpalika Shopping Center,New Nagar Road, 02425-226981/82 AHMEDNAGAR SANGAMNER Shubham Computers Yogesh Bhagwat 9822069547 Sangamner, Tal. Sangamner, Dist /7588025925 Ahmednagar Opposite OLD Nagarpalika AHMEDNAGAR KOPARGAON Cybernet Systems Shrikant Joshi 02423-222366 / 223566 9763715766 Building,Kopargaon – 423601 Near Bus Stand, Behind Hotel Prashant, AHMEDNAGAR AKOLE Media Infotech Sudhir Fargade 02424-222200 7387112323 Akole, Tal Akole Dist Ahmadnagar K V Road ,Near Anupam photo studio W 02422-226933 / AHMEDNAGAR SHRIRAMPUR Manik Computers Sachin SONI 9763715750 NO 6 ,Shrirampur 9850031828 HI-TECH Computer -

Village Map Taluka: Karmala District: Solapur
Shrigonda Village Map Jamkhed Taluka: Karmala District: Solapur !( Khadaki Padali Punwar Jategaon Aljapur Karjat Taratgaon Kamone Limbewadi Wadgaon (N) Bitargaon (Shrigonde) µ Ghargaon Balewadi 4.5 2.25 0 4.5 9 13.5 Mangi Ravgaon Potegaon km Wadgaon Kh. Pothare Nilaj Paranda Borgaon Vanjarwadi BhoseHiwarwadi Wadachiwadi Dilmeshwar Gorewadi Karmala (Rural)DhaykhindiKhambewadi Location Index Pimpalwadi KARMALA Morwad Hulgewadi Roshewadi !( Devichamal Karmala (M Cl) Karanje District Index Korti Bhalewadi Nandurbar Veet Pande Mirghavan Bhandara Dhule Amravati Nagpur Gondiya Savadi Vihal Jalgaon Gharatwadi Deolali Akola Wardha Arjunnagar Buldana Kumbhargaon Kuskarwadi Nashik Washim Chandrapur Yavatmal Pondhvadi Hivare Kolgaon Palghar Aurangabad Anjandoh Jalna Hingoli Gadchiroli Kawalwadi Gulsadi Thane Ahmednagar Parbhani Bhilarwadi Hisare Mumbai Suburban Nanded Daund Shelgaon (K) Nimgaon (H) Bid Delwadi Khadakewadi Mumbai Divegavan Rajuri Phisare Pune Zare Raigarh Bidar Ramwadi Manjargaon Latur Gaundare Osmanabad Umrad Domgaon Sarapdoh Sounde Bhagatwadi Hingani Satara Solapur Jinnti Ritewadi Ratnagiri Parewadi Kumbhej Salse Awati Sangli Undargaon Gulmarwadi Maharashtra State Pophalaj Kolhapur Katraj Sade Kondhar Chincholi Sindhudurg Washibe Warkatne Jehurwadi Kondhej Dharwad Pomalwadi Sogaon Kedgaon Alsunde Khatgaon Ketur Nerle Takali (Rashin) Shetphal Jeur Goyegaon Taluka Index Lavhe Warkute Ghoti Nimbhore Karmala Dahigaon Chikhalthan Shelgaon (Wangi) Barshi Kugaon Pathurdi Madha Wangi Bhalavni Malwadi Wangi Malshiras Mohol Pangare Wangi -

Review of Research Journal:International Monthly Scholarly
ISSN 2249-894X Impact Factor : 3.1402 (UIF) Volume - 5 | Issue - 3 | Dec - 2015 Review Of Research _________________________________________________________________________________ SOCIAL AUDIT OF NATIONAL RURAL EMPLOYMENT GUARANTEE SCHEME IN SOLAPUR DISTRICT Dr. S. V. Shinde Associate Professor , D. A. V. Velankar College of Commerce, Solapur. ABSTRACT : INTRODUCTION : National Rural Employment Guarantee Act 2005 (or, NREGA The act was first No 42, later renamed as the "Mahatma Gandhi National Rural proposed in 1991 by Narasimha Employment Guarantee Act", MGNREGA), is an Indian labour law Rao. In 2006, it was finally and social security measure that aims to guarantee the 'right to work'. accepted in the parliament and It aims to enhance livelihood security in rural areas by commenced implementation in providing at least 100 days of wage employment in a financial year to 200 districts of India. Based on every household whose adult members volunteer to do unskilled this pilot experience, NREGA manual work. was scoped up to covered all the districts of India from 1 April 2008. The MGNREGA was initiated with the objective of "enhancing livelihood security inrural areas by providing at least 100 days of guaranteed wage employment in a financial year, to every household whose adult members volunteer to do unskilled manual work". Another aim of MGNREGA is to create durable assets (such as roads, canals, ponds, wells). Employment is to be provided within 5 km of an applicant's residence, and minimum wages are to be paid. If work is not provided within 15 days of applying, applicants are entitled to an unemployment allowance. Thus, employment under MGNREGA is a legal entitlement. -
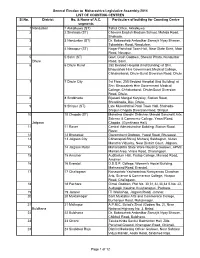
V E 2014 Counting Centres
General Election to Maharashtra Legislative Assembly-2014 LIST OF COUNTING CENTRES Sl.No. District No. & Name of A.C. Particulars of building for Counting Centre segments 1 Nandurbar 1 Akkalkuwa (ST) Tahsil Office, Akkalkuwa 2 2 Shahada (ST) Chavara English Medium School, Mohida Road, Shahada. 3 3 Nandurbar (ST) Dr. Babasaheb Ambedkar Samajik Nyay Bhavan, Tokartalav Road, Nandurbar. 4 4 Nawapur (ST) Nagar Parishad Town Hall, Near State Bank, Main Road, Navapur. 5 5 Sakri (ST) Govt. Grain Godown, Shewali Phata, Nandurbar Dhule Road, Sakri. 6 6 Dhule Rural 250 Bedded Hospital (Iind Building) at Shri. Bhausaheb Hire Government Medical College, Chhakarbardi, Dhule-Surat Diversion Road, Dhule. 7 7 Dhule City 1st Floor, 250 Bedded Hospital (Iind Building) at Shri. Bhausaheb Hire Government Medical College, Chhakarbardi, Dhule-Surat Diversion Road, Dhule. 8 8 Sindkheda Bijasani Mangal Karyalay, Station Road, Shindkheda, Dist. Dhule. 9 9 Shirpur (ST) Late Mukeshbhai Patel Town Hall, Shahada- Shirpur-Chopda Diversion Road, Shirpur. 10 10 Chopda (ST) Mahatma Gandhi Shikshan Mandal Sanchalit Arts, Science & Commerce College, Yawal Road, Jalgaon Chopda. (Gymkhana Hall) 11 11 Raver Central Administrative Building, Station Road, Raver. 12 12 Bhusawal Government Godown, Yawal Road, Bhusawal. 13 13 Jalgaon City Chhatrapati Shivaji Maharaj Sabhagruh, Nutan Maratha Vidyalay, Near District Court, Jalgaon. 14 14 Jalgaon Rural Maharashtra State Ware Housing Godown, APMC Market Area, Virare Road, Dharangaon. 15 15 Amalner Auditorium Hall, Pratap College, Marwad Road, Amalner. 16 16 Erandol D.D.S.P. College, Women's Hostel Building, Mahsawad Road, Erandol. 17 17 Chalisgaon Nanasaheb Yashwantrao Narayanrao Chawhan Arts, Science & Commerece College, Hirapur Road, Chalisgaon. -

Annexure 1 Compound Growth Rate of Area and Yield in Study Area 1
Annexure 1 Compound Growth Rate of area and yield in study area 1. Rabi Sorghum Area Taluka District CGR r Significant change Jamkhed Ahmadnagar 0.01 0.75 0.001 level + Karjat Ahmadnagar 0 -0.07 NS Nagar Ahmadnagar 0 -0.03 NS Nevasa Ahmadnagar -0.01 -0.87 0.001 level - Parner Ahmadnagar 0 0.38 NS Pathardi Ahmadnagar 0 0.46 0.05 level + Rahuri Ahmadnagar -0.01 -0.44 0.05 level - Shevgaon Ahmadnagar -0.02 -0.8 0.001 level - Shrigonda Ahmadnagar 0 -0.11 NS Ambejogai Beed -0.03 -0.6 0.01 level - Ashti Beed Beed 0.01 0.33 NS Beed Beed 0.01 0.73 0.001 level + Dharur Beed 0 -0.03 NS Georai Beed 0.01 0.42 0.05 level + Kaij Beed -0.01 -0.39 0.05 level - Manjlegaon Beed -0.01 -0.48 0.05 level - Parli Beed -0.04 -0.49 NS Patoda Beed -0.01 -0.43 0.05 level - Shirur (Kasar) Beed 0.04 1 NS Wadwani Beed 0 1 NS Bhum Osmanabad -0.01 -0.26 NS Kalamb Osmanabad 0.01 0.26 NS Lohara Osmanabad 0 -0.6 NS Osmanabad Osmanabad 0.01 0.59 0.01 level + Paranda Osmanabad 0.01 0.5 0.05 level + Tuljapur Osmanabad 0 0.12 NS Umarga Osmanabad 0 0.05 NS Washi Osmanabad 0.05 1 NS Baramati Pune 0 0.13 NS Daund Pune 0 -0.35 NS Indapur Pune 0 -0.31 NS Man Satara 0 -0.24 NS Phaltan Satara -0.01 -0.58 0.01 level - Akkalkot Solapur 0 -0.25 NS Barshi Solapur 0 -0.43 0.05 level - Karmala Solapur 0 -0.36 NS Madha Solapur 0 -0.1 NS Malshiras Solapur -0.01 -0.37 NS Mangalvedhe Solapur 0 0.3 NS Mohol Solapur 0 -0.09 NS Pandharpur Solapur -0.01 -0.36 NS Sangole Solapur 0 -0.32 NS Solapur North Solapur 0 -0.2 NS Solapur South Solapur 0 -0.22 NS CGR= Compound rate of growth; r= correlation coefficient; NS = not significant 2. -

Village Map Madha Taluka: Pandharpur Kanhapuri District: Solapur
Indapur Village Map Madha Taluka: Pandharpur Kanhapuri District: Solapur Umbare Jaloli Karole Sangavi Karkamb Badalkot Bardi µ Malshiras 3.5 1.75 0 3.5 7 10.5 Nematwadi km Nandore Pehe Jadhavwadi Kharatwadi Mohol Avhe Sugavabhose (N.V.)Pandharewadi Bhose Ujani (N.V.) Shevate Location Index Taratgaon (Bhose) Mendhapur Ropale Patvardhan Kuroli District Index Chilaiwadi Nandurbar Ajote (N.V.) Bhandara Khed Bhose Devade Dhule Amravati Nagpur Gondiya Jalgaon Sugavakhurd (N.V.) Babhulgaon Akola Wardha Hole Takaligursala (N.V.) Buldana Tungat Nashik Washim Chandrapur Pirachi Kuroli Yavatmal Palghar Aurangabad Jalna Gadchiroli Narayan Chincholi Hingoli Thane Ahmednagar Parbhani Khed Bhalawani Venunagar Mumbai Suburban Nanded Shelve Magarwadi Bid Wadi Kuroli Kauthali Mumbai Keskarwadi Adhiv Raigarh Pune Shendgewadi Latur Bidar Gurasale Ishwar Wathar Osmanabad Solapur Suste Satara Degaon Shirthon Tarapur Ratnagiri Dhondewadi Bhandi Shegaon Sangli Chincholi BhoseBhatumbare Bitargaon (N.V.) Maharashtra State Sharadrenagar(N.V.) Kolhapur Bhalawani Ajansond Fulchincholi Sindhudurg Wakhari Dharwad Supli PANDHARPUR Isbavi !( Shegaon Dumala Chale Jainwadi Mundhewadi Upari Kharsoli Pandharpur (M Cl) Puluj Taluka Index Vite Gopalpur Kondharki Gadegaon Karmala Pohargaon Palshi Pulujwadi Takli Barshi Gardi Korty Madha Kothale Ranzani Ambe Sarkoli Shankargaon Lonarwadi Sonke Anawali Malshiras Mohol Pandharpur Solapur North Ambechincholi Nali Bohali Kasegaon Ozewadi Eklaspur Nepatgaon Solapur South Tisangi ShirgaonTaratgaon Kasegaon Akkalkot Sangole Mangalvedhe Legend Umbargaon Siddhewadi !( Taluka Head Quarter Khardi Chinchumbe District: Solapur Railway Tavashi National Highway Sangole Village maps from Land Record Department, GoM. Tanali Data Source: State Highway Shetphal Waterbody/River from Satellite Imagery. Mangalvedhe State Boundary Generated By: District Boundary Maharashtra Remote Sensing Applications Centre Taluka Boundary !( Autonomous Body of Planning Department, Government of Maharashtra, VNIT Campus, Village Boundary South Am bazari Road, Nagpur 440 010. -

Village Map Taluka: Barshi District: Solapur
!( Village Map Washi Koregaon Taluka: Barshi Bhum District: Solapur Kalamb Khadkoni Chumb Dhamangaon (A) Bhansale Kalambawadi (A) Kategaon Walwad Chare Pimpalwadi Umbarge Ukadgaon Wagachiwadi (N.V.) Borgaon [Kh] Shirale µ Mandegaon 4.5 2.25 0 4.5 9 13.5 Agalgaon Pathari Pimpalgaon (Dhas)Khadkalgaon Babhulgaon Pandhari km Belgaon Dhebarewadi (N.V.) Dhanore Pangri Paranda DevgaonShelgaon (Vhale)Tadsoudne Chincholi Kuslamb Ghari Gholvewadi Bhoyare Wanewadi Puri Kandalgaon Gadegaon Jahanpur Location Index Gatachiwadi DhotrePimpalgaon (Pangri) Jamgaon (Agalgaon) Mamdapur Nagobachiwadi Arangaon District Index Nandurbar Kari BARSHI Gormale Osmanabad Bhandara Barshi (M!( Cl) Khamgaon Yelamb Phaphalwadi Tawadi Dhule Amravati Nagpur Gondiya Lakshyachiwadi Jalgaon Akola Wardha Barshi Buldana Shelgaon (Markad) Upalai (Thonge) Nashik Washim Chandrapur Tandulwadi Yavatmal Alipur Chikharde Palghar Aurangabad Nari (Bhandewadi) Jalna Hingoli Gadchiroli Kasarwadi Thane Ahmednagar Parbhani Nariwadi Mumbai Suburban Nanded Saundare Mahagaon Mumbai Bid Balewadi Indapur Pune Bavi Raigarh Bidar Godasewadi (N.V.) Latur Khandavi Sawargaon Osmanabad Wangarwadi Malegaon Kapasi Dadshinge Satara Solapur Kalambwadi (P) Tambewadi Shendri Kavhe Ratnagiri Pimpalgaon (Pangaon) Sangli Jamgaon (P) Bhatambare Pimpri (Ratanjan) Maharashtra State Kolhapur Hingani (Pangaon) Sindhudurg Korfale Pangaon Borgaon (Zadi) Shripat Pimpri Sakat Dharwad Bhoinje Pimpari (Pangaon) Nandani Upale (Dumala) Zadi Part of Paranda Taluka Halduge Taluka Index Rastapur Ghanegaon Zaregaon Karmala -
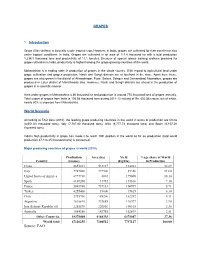
Source: FAO Indian Scenario
GRAPES 1. Introduction Grape (Vitis vinifera) is basically a sub- tropical crop. However, in India, grapes are cultivated for their excellence also under tropical conditions. In India, Grapes are cultivated in an area of 111.4 thousand ha with a total production 1,234.9 thousand tons and productivity of 11.1 tons/ha. Because of special arbour training systems provided for grape cultivation in India, productivity is highest among the grape growing countries of the world. Maharashtra is a leading state in production of grapes in the whole country. With regard to agricultural land under grape cultivation and grapes production, Nasik and Sangli districts are at forefront in the state. Apart from these, grapes are also grown in the district of Ahmednagar, Pune, Satara, Solapur and Osmanabad. Nowadays, grapes are produced in Latur district of Marathwada also. However, Nasik and Sangli districts are ahead in the production of grapes in a scientific manner. Area under grapes in Maharashtra is 86 thousand ha and production is around 774 thousand tons of grapes annually. Total export of grapes from India is 108.58 thousand tons during 2011-12 valuing of Rs. 602.88 crores, out of which, nearly 80% is exported from Maharashtra. World Scenario According to FAO data (2010), the leading grape producing countries in the world in terms of production are China (8,651.83 thousand tons), Italy (7,787.80 thousand tons), USA (6,777.73 thousand tons) and Spain (6,107.20 thousand tons). India’s high productivity in grape has made it to reach 18th position in the world as far as production (total world production 67,116.25 thousand tons) is concerned. -

Mar/Apr 2015 Course Part: Fe-I & Fe-Ii & Se-Ii College
SOLAPUR UNIVERSITY Collegewise Students' Name List EXAM: MAR/APR 2015 COURSE PART: FE-I & FE-II & SE-II COLLEGE: (8) - WALCHAND INSTITUTE OF TECHNOLOGY, SOLAPUR BRANCH: CIVIL ENGINEERING -------------------------------------------------------------------------------- SEAT PRN STUDENT'S CENTER NO. NO. NAME NAME -------------------------------------------------------------------------------- EM-I, EG, EM-II(N), ENVSCI, ENVSCI, SM(N), SUR(N), BCD(N), FM(N), WRE(N), EM-III(N), CP&NM(N) 832007 1100006383 SINDGI NINAD MILIND MANISHA WIT WIT->WALCHAND INSTITUTE OF TECHNOLOGY, SOLAPUR Total Number Of Students :1 Note : If there is any mistakes in the name & subject(s), please communicate to this office immediately. -------------------------------------------------------------------------------- 15/04/2015 PAGE NO. 1 SOLAPUR UNIVERSITY Collegewise Students' Name List EXAM: MAR/APR 2015 COURSE PART: FE-I & SE-I & SE-II COLLEGE: (8) - WALCHAND INSTITUTE OF TECHNOLOGY, SOLAPUR BRANCH: CIVIL ENGINEERING -------------------------------------------------------------------------------- SEAT PRN STUDENT'S CENTER NO. NO. NAME NAME -------------------------------------------------------------------------------- EP-I, CT(N), SM-I(N), S-I(N), BCD (N), FM-I(N), EG(N), ENVSCI, ENVSCI, SM(N), SUR(N), BCD(N), FM(N), WRE(N), EM-III(N), CP&NM(N) 832009 1100006361 KAMBLE PRASHANT MOHAN SHAILA WIT WIT->WALCHAND INSTITUTE OF TECHNOLOGY, SOLAPUR Total Number Of Students :2 Note : If there is any mistakes in the name & subject(s), please communicate to this office immediately. -

Village Map Taluka: Malshiras District: Solapur Madha
Karmala Baramati Village Map Taluka: Malshiras District: Solapur Madha Kurbavi Indapur Deshmukhwadi Ekshiv Shindewadi Tambewadi Kalamboli Dombalwadi Hanumanwadi Bangarde Palasmandal Pirale Phaltan Dharmpuri µ Dahigaon 4.5 2.25 0 4.5 9 13.5 Gursale Motewadi Sangam Babhulgaon km Kadamwadi Wafegaon Umbare Dahigaon Ganeshgaon Fondshiras Pratapnagar (N.V.) Tirwandi Tambave Morochi Chakore Bijvadi Wagholi Markadwadi Kondabavi Anandnagar Location Index Karunde Natepute (CT) Tamsidwadi Savatgavhan Lawang Medad Kacharewadi Sangramnagar (N.V.) (CT) Akluj (CT) Bagechiwadi Vatpali (N.V.) District Index Malinagar Pimpari Nandurbar Purandawade Bhandara Yashawantnagar Mahalung Mandave Sadashivnagar Yeliv MALSHIRAS Girzani Malewadi Umbare (Velapur) Dhule Amravati Nagpur Gondiya !(Malshiras Jalgaon Kothale Mire Akola Wardha Buldana Shiwarvasti (N.V.) Paniv Chaundeshwarwadi Kondarpatta Nashik Washim Chandrapur Lonand Yavatmal Jadhavwadi Neware Dhulenagar N.V.) Vijaywadi Palghar Aurangabad Londhe Mohitewadi Jalna Gadchiroli Khandali Hingoli Bhamburdi Borgaon Thane Dattanagar Ahmednagar Parbhani Fadtari Dombalwadi Vizori Malewadi Mumbai Suburban Nanded Jambud Mumbai Bid Giravi Motewadi Pune Raigarh Bidar Kanher Khudus Latur Malkhambi Osmanabad Islampur Ughadewadi Nitavewadi (N.V.) Vithalwadi Solapur Zunjewadi Pisewadi Satara Ratnagiri Bhamb Sangli Velapur Bondale Khalawe Maharashtra State Rede Mandaki Goradwadi Kolhapur Tarangfal Sindhudurg Nimgaon Dharwad Dasur Tondale Dhanore Jalbhavi Taluka Index Shendechinch Chandapuri Magarwadi (N.V.) Pandharpur Karmala -

Indian Streams Research Journal
Vol 5 Issue 5 June 2015 ISSN No : 2230-7850 ORIGINAL ARTICLE International Multidisciplinary Research Journal Indian Streams Research Journal Executive Editor Editor-in-Chief Ashok Yakkaldevi H.N.Jagtap Welcome to ISRJ RNI MAHMUL/2011/38595 ISSN No.2230-7850 Indian Streams Research Journal is a multidisciplinary research journal, published monthly in English, Hindi & Marathi Language. All research papers submitted to the journal will be double - blind peer reviewed referred by members of the editorial board.Readers will include investigator in universities, research institutes government and industry with research interest in the general subjects. International Advisory Board Flávio de São Pedro Filho Mohammad Hailat Hasan Baktir Federal University of Rondonia, Brazil Dept. of Mathematical Sciences, English Language and Literature University of South Carolina Aiken Department, Kayseri Kamani Perera Regional Center For Strategic Studies, Sri Abdullah Sabbagh Ghayoor Abbas Chotana Lanka Engineering Studies, Sydney Dept of Chemistry, Lahore University of Management Sciences[PK] Janaki Sinnasamy Ecaterina Patrascu Librarian, University of Malaya Spiru Haret University, Bucharest Anna Maria Constantinovici AL. I. Cuza University, Romania Romona Mihaila Loredana Bosca Spiru Haret University, Romania Spiru Haret University, Romania Ilie Pintea, Spiru Haret University, Romania Delia Serbescu Fabricio Moraes de Almeida Spiru Haret University, Bucharest, Federal University of Rondonia, Brazil Xiaohua Yang Romania PhD, USA George - Calin SERITAN Anurag Misra Faculty of Philosophy and Socio-Political ......More DBS College, Kanpur Sciences Al. I. Cuza University, Iasi Titus PopPhD, Partium Christian University, Oradea,Romania Editorial Board Pratap Vyamktrao Naikwade Iresh Swami Rajendra Shendge ASP College Devrukh,Ratnagiri,MS India Ex - VC. Solapur University, Solapur Director, B.C.U.D. -

General Election to Maharashtra Legislative Assembly 2019
General Election to Maharashtra Legislative Assembly 2019 Returning officer for Assembly Constituencies District Name and no.of AC Designation of Returning Name of Returning Phone No. E-mail Officer Officer Nandurbar 1 – Akkalkuwa (ST) Sub Divisional Officer, Shri. Kailas Kadlag 02567-232373 [email protected] Taloda Nandurbar 2 – Shahada (ST) Sub Divisional Officer, Shri. Chetan Girase 02565-227733 [email protected] Shahada Nandurbar 3 – Nandurbar (ST) Sub Divisional Officer, Shri. M.T. Sudhalkar 02564-210010 [email protected] Nandurbar Nandurbar 4 – Nawapur (ST) District Supply officer, Shri. Mahesh Shelar 02564-210009 [email protected] Nandurbar Dhule 5 – Sakri (ST) Deputy Collector (EGS), Shri. Govinda Danej 2562-288709 [email protected] Dhule. Dhule 6 – Dhule Rural Sub Divisional Officer, Shri. Bhimrao Darade 2562-288719 [email protected] Dhule Division Dhule Dhule 7 – Dhule City Deputy Collector ( Land Shri. Shrikumar Chichkar 2562-234711 [email protected] Acquisition ), no.1, Dhule Dhule 8 – Sindkheda Deputy Collector ( Land Smt. Surekha Chawan 2562-232167 [email protected] Acquisition ), (MIP), Dhule Dhule 9 – Shirpur (ST) Sub Divisional Officer, Shri. Vikram Bandal 2563-256397 [email protected] Shirpur Jalgaon 10 – Chopda (ST) Deputy Collector Smt. Shubhangi Bharde 02586-220076 [email protected] (Resettlement) Jalgaon Jalgaon 11 – Raver Sub Divisional Officer, shri. Ajit Thorbole 02585-246555/ 02584- [email protected] Faizpur 250226 Jalgaon 12 – Bhusawal (SC) Sub Divisional Officer, Shri. Ramsin Sulane 02582-23191/ 02584- [email protected] Bhusawal 222592 Jalgaon 13 – Jalgaon City Sub Divisional Officer, Smt. Dipmala Chaure 0257-2220868 [email protected] Jalgaon Jalgaon 14 – Jalgaon Rural Deputy Collector ( Land Shri.