C H a R a C T E R I Z a T I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Notification
THE WEST BENGAL COLLEGE SERVICE COMMISSION NOTICE FOR REQUISITION AGAINST VACANCY FOR THE POST OF PRINCIPAL __________________________________________________________________________________ The Authorities of all the Government-aided General Degree Colleges in West Bengal are hereby requested to submit the Requisition (in duplicate) in the prescribed format along with all the necessary documents (see note below) within 16/12/2019 against the vacancy for the post of Principal created by way of retirement, resignation, death, dismissal or approval of a new post by the Government. It is further requested to check the enlisted vacancy status (annexed herewith) for the post of Principal and discrepancy, if any, be brought to the notice of the Commission immediately. Note : Requisition (using PROFORMA 2019) along with Annexures must be submitted in two sets. Requisite Annexures : i) Relevant G.B resolution and ii) Copy of the G.O., if the post is newly created. Date : 30/10/2019 By order Controller of Examinations Please see next pages for VACANCY STATUS & REQUISITION FORM __________________________________________________________________________________ PROFORMA 2019 (PRINCIPAL) THE WEST BENGAL COLLEGE SERVICE COMMISSION REQUISITION FORM TO BE USED BY COLLEGES FOR INTIMATING VACANCIES FOR THE POST OF PRINCIPAL 1. a) Name of the College : b) Address with PIN CODE and Contact No. : 2. Name of the TIC with contact No. : 3. Affiliating University : 4. Name of the Post : PRINCIPAL (Please attach a separate sheet stating the subjects taught in the College) 5. Reason for creation of vacancy (Retirement/Resignation/ : Death/Dismissal/New Post) 6. a) If new post, G.O. No. of creation of new post : b) Otherwise, name of the previous incumbent : 7. -

Sl No Name of the Participant College/University 1 Saurav Dasthakur Visva-Bharati University 2 Amrita Biswas Hiralal Bhakat Coll
UGC-HUMAN RESOURCE DEVELOPMENT CENTRE THE UNIVERSITY OF BURDWAN LIST OF TEACHER PARTICIPANTS OF ACADEMIC YEAR 2015-2016 6TH RC IN WOMEN STUDIES 02-06-15 TO 22-06-15 Sl No Name of the Participant College/University 1 Saurav Dasthakur Visva-Bharati University Hiralal Bhakat College, The University 2 Amrita Biswas of Burdwan Sambhu Nath College, The University 3 Chhanda Das Gupta (Nayek) of Burdwan Sambhu Nath College, The University 4 Pradip Biswas of Burdwan 5 Sachindra Nath Mandal Moyna College, Vidyasagar University S.C.B.C College, Lalbagh, 6 Ranjusree Sarkar Murshidabad, Kalyani University Ramnagar College, Depal, Vidyasagar 7 Dr. Sutanu Kumar Mahapatra University Ramnagar College, Depal, Vidyasagar 8 Mukteswar Das University Victoria Institution (College), Calcutta 9 Simanti Bandyopadhyay University B.B. College, Asansol, The University 10 Kakali Kundu of Burdwan T.D.B College, Raniganj, The 11 Pradip Kumar Chakraborty University of Burdwan Union Christian Training College, 12 Saswati Roy Kalyani University Bir Lachit Borphukan College, 13 Shri Lakshi Sensua Dibrugarh University Bir Lachit Borphukan College, 14 Ajit Borah Dibrugarh University Bir Lachit Borphukan College, 15 Purna Phulkonwar Dibrugarh University Asannagar M.M.T. College, University 16 Nilendu Biswas of Kalyani Chapra Bangalihi Mahavidyalaya, 17 Gargi Sengupta Kalyani University Kashipur Michael Madhusudhan 18 Bignanananda Mukhopadhyay Mahavidyalaya, Sidho-Kanho-Birsa University Samsi College, University of Gour 19 Dr. Pralay Kanti Ghosh Banga Abhedananda Mahavidyalaya, The 20 Shiben Kumar Sarkar University of Burdwan Sl No Name of the Participant College/University Rampurhat College, The University of 21 Dr. Prabal Kumar Sinha Burdwan Bankura Christian College, The 22 Mousumi Das University of Burdwan M.U.C. -
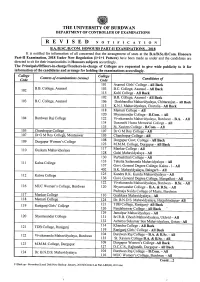
REVISED NOTIFICATION B.A.IB.SC.Lb.COM
THE UNIVERSITY OF BURDWAN DEPARTMENT OF CONTROLLER OF EXAMINATIONS REVISED NOTIFICATION B.A.IB.SC.lB.COM. HONOURS PART-II EXAMINATIONS - 2018 It is notified for information of all concerned that the arrangement of seats at the B.AIB.Sc.IB.Com. Honours Part-II Examination, 2018 Under New Regulation (1+1+1 Pattern) have been made as under and the candidates are directed to sit for their 'examinatiohs in Honours subjects accordingly. ( The Principals/Officers-in-chargeffeachers-in-charge of Colleges are requested to give wide publicity to it for information of the candidates and arrange for holding the examinations accordingly. College College Centres of examinations (venue) Code Code Candidates of 101 Asansol Girls' College - All Back 102 B.B. College, Asansol 103 B.C. College, Asansol- All Back 115 Kulti College - All Back 102 B.B. College, Asansol - All Back 103 B.C. College, Asansol 106 Deshbandhu Mahavidyalaya, Chittaranjan - All Back 113 K.N.I. Mahavidyalaya, Churulia - All Back 118 Memari College - All 120 Shyamsundar College - B.Com. - All 104 Burdwan Raj College 122 Vivekananda Mahavidyalaya, Burdwan - B.A. - All 134 Dasarathi Hazra Memorial College - All 135 St. Xaviers College - B.Com. - All 105 Chandrapur College 107 Dr G M Roy College - All 107 Dr G M Roy College, Monteswar 105 Chandrapur College - All 109 Durgapur Women's College 108 Durgapur Govt. College - All Back 123 M.M.M. College, Durgapur - All Back 110 Guskara Mahavidyalaya 117 Mankar College - All 128 Galsi Mahavidyalaya - All 130 Purbashthali College - All 111 Kalna College 133 Tehatta Sadananda Mahavidyalaya - All 137 Govt. -

State Aided College Teacher Department: Botany Ramananda College, Bishnupur Bankura, West Bengal, India
Dr. Rebeca Ghanta M.Sc., Ph.D. Designation: State Aided College Teacher Department: Botany Ramananda College, Bishnupur Bankura, West Bengal, India E-mail: [email protected] AREAS OF INTEREST/SPECIALISATION • Mycology and Plant pathology ACADEMIC ACHIEVEMENTS • M.Sc. (2005, CSJMU, Kanpur) • Ph.D. (2014, The University of Burdwan) • B.Ed. (2015, The University of Burdwan) RESEARCH EXPERIENCE From To Name and Address of Funding Position held Agency / Organization September 2008 April 2013 The University of Burdwan Project Fellow ACADEMIC EXPERIENCE • January 2020 – Till Date : State Aided College Teacher-I at Ramananda College, Bishnupur 722122 o Theoretical and practical courses in Botany (UG & PG). o Performs Exam related duties assigned by Bankura University in all respect o Development of students’ curriculum. • August 2018-December 2019 : Guest Lecturer in Botany at Ramananda College, Bishnupur 722122 o Theoretical and practical courses in Botany (UG & PG). o Performs Exam related duties assigned by Bankura University in all respect o Development of students’ curriculum. • August 2016- August 2018: Guest Lecturer in Botany at Indas Mahavidyalaya, Indas 722205 o Theoretical and practical courses in Botany. • September 2013-February 2014 : Guest Lecturer in Botany at P. D.Women’s College, Jalpaiguri 735101 o Theoretical and practical courses in Botany. o Development of students’ curriculum. • August 2006– March 2008 : Part Time Lecturer at Bankura Christian College, Bankura 722101, India o Theoretical and practical courses in Botany. o Development of students’ curriculum. ADMINISTRATIVE EXPERIENCE • Admission related work • Member of Research Committee PUBLICATIONS (List of Journals/Proceedings/Chapter in Books) Year 2016 • Ghanta, R., Sen, M., Mukhopadhyay, R. and Dutta, S. -

THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Post of Principal in Govt
THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Post of Principal in Govt. - Aided General Degree Colleges (Advt. No. 2/2019) SL.NO. NAME OF THE COLLEGES UNIVERSITY 1 Birsha Munda Memorial College 2 Chhatna Chandidas Mahavidyalaya 3 Indas Mahavidyalaya 4 Khatra Adibasi Mahavidyalaya BANKURA UNIVERSITY 5 P. R. M. S. Mahavidyalaya 6 Raipur Block Mahavidyalaya 7 Saltora Netaji Centenary College 8 Abhedananda Mahavidyalaya 9 Dr. Bhupendranath Dutta Smriti Mahavidyalaya 10 Hiralal Bhakat College 11 M.U.C. Women's College 12 Raja Rammohan Roy Mahavidyalaya 13 Rajnagar Mahavidyalaya BURDWAN UNIVERSITY 14 Rampurhat College 15 Sailajananda Falguni Smriti Mahavidyalaya 16 Sree Gopal Banerjee College 17 Sri Ramkrishna Sarada Vidyamahapitha 18 Tehatta Sadananda Mahavidyalaya 19 Bakshirhat Mahavidyalaya 20 Baneswar Sarathibala Mahavidyayala 21 Dinhata College 22 Ghoksadanga Birendra Mahavidyalaya CPB University 23 Mathabhanga College 24 Mekliganj College 25 Thakur Panchanan Mahila Mahavidyalaya THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Post of Principal in Govt. - Aided General Degree Colleges (Advt. No. 2/2019) SL.NO. NAME OF THE COLLEGES UNIVERSITY 26 Bangabasi Morning College 27 Gangadharpur Mahavidyamandir 28 Netaji Nagar College 29 Pathar Pratima Mahavidyalaya 30 Rani Birla Girls' College 31 Sagar Mahavidyalaya 32 Saheed Anurupchandra Mahavidyalaya CALCUTTA UNIVERSITY 33 Seth Soorajmull Jalan Girls' College 34 Sibani Mandal Mahavidyalaya 35 Sukanta College 36 South Calcutta Law College 37 Surendranath Evening College 38 Surendranath Law College 39 Sundarban Mahavidyalaya 40 Chanchal College 41 Dewan Abdul Gani College 42 Dr. Meghnad Saha College 43 Gangarampur College 44 Harishchandrapur College GOUR BANGA UNIVERSITY 45 Jamini Mazumder Memorial College 46 Nathaniyal Murmu Memorial College 47 Pakuahat Degree College 48 Samsi College 49 South Malda College THE WEST BENGAL COLLEGE SERVICE COMMISSION Vacancy Status for the Post of Principal in Govt. -

61 20 20 21 Bankura University
BANKURA UNIVERSITY Department of Controller of Examinations Programme for UG SEM-III Practical Examinations, 2018-19 Subject: Mathematics (Core P-7) Venue: Department of Mathematics, Bankura Christian College, PO+Dist: Bankura No. of Date Time UID Examinee 17633121001 17633121002 17633121004 17633121005 17633121006 17633121007 17633121008 17633121009 11.00 Noon 2/18/2019 to 17633121010 17633121011 17633121013 17633121014 17633121015 17633121016 17633121017 17633121018 20 01.00 PM 17633121019 17633121020 17633121021 17633121022 17633121023 17633121024 17633121027 17633121028 17633121029 17633121030 17633121031 17633121032 02.00 PM 2/18/2019 to 17633121033 17633121034 17633121035 17633121036 17633121038 17633121039 17633121040 17633121041 20 04.00 PM 17633121044 17633121046 17633121048 17633121052 17633121054 17633121055 17633121057 17633121058 17633121059 17633121060 17633121062 17633121063 11.00 Noon 2/19/2019 to 17633121064 17633121065 17633121066 17633121067 17633121068 17633121069 17633121070 17633121071 21 01.00 PM 17633121072 17633121073 17633121074 17633121075 17633121076 Total: 61 Copy forwarded to The Principal, Bankura Sammilani College, Bankura for information with the request to give wide circulation among the candidates concerned for proper guidance. Controller of Examinations BANKURA UNIVERSITY Department of Controller of Examinations Programme for UG SEM-III Practical Examinations, 2018-19 Subject: Mathematics (Core P-7) Venue: Department of Mathematics, Bankura Christian College, PO+Dist: Bankura No. of Date Time UID Examinee -

SEAT ALLOTMENT.Xlsx
BANKURA UNIVERSITY Center Allotment for Honours Courses_BKU__UG_Sem II_2017-18 CENTER COLLEGE CENTER NAME COLLEGE NAME CODE CODE 102 BANKURA SAMMILANI COLLEGE 101 BANKURA CHRISTIAN COLLEGE 103 BANKURA ZILLA SARADAMANI MAHILA MAHAVIDYAPITH 112 ONDA THANA MAHAVIDYALAYA 102 BANKURA SAMMILANI COLLEGE 101 BANKURA CHRISTIAN COLLEGE 107 CHHATNA CHANDIDAS MAHAVIDYALAYA BANKURA ZILLA SARADAMANI 103 108 GOBINDAPRASAD MAHAVIDYALAYA MAHILA MAHAVIDYAPITH 110 JAMINI ROY COLLEGE 119 SALTORA NETAJI CENTENARY COLLEGE 104 BARJORA COLLEGE 122 KABI JAGADRAM ROY GOVERNMENT GENERAL DEGREE COLLEGE, MEJIA 118 SALDIHA COLLEGE 111 KHATRA ADIBASI MAHAVIDYALAYA 123 GOVT. GENERAL DEGREE COLLEGE, RANIBANDH 105 BIRSHA MUNDA MEMORIAL COLLEGE 113 PANCHMURA MAHAVIDYALAYA 114 PANDIT RAGHUNATH MURMU SMRITI MAHAVIDYALAYA 116 RAIPUR BLOCK MAHAVIDYALAYA 106 CHATRA RAMAI PANDIT MAHAVIDYALAYA 109 INDAS MAHAVIDYALAYA 117 RAMANANDA COLLEGE 113 PANCHMURA MAHAVIDYALAYA 115 PATRASAYER MAHAVIDYALAYA 120 SONAMUKHI COLLEGE 118 SALDIHA COLLEGE 111 KHATRA ADIBASI MAHAVIDYALAYA 104 BARJORA COLLEGE 120 SONAMUKHI COLLEGE 117 RAMANANDA COLLEGE 121 SWAMI DHANANJOY DAS KATHIABABA MAHAVIDYALAYA BANKURA UNIVERSITY Center Allotment for Programme Courses_BKU__UG_Sem II_2017-18 CENTRE NAME COLLEGE NAME CENTRECODE COLLEGECODE 102 BANKURA SAMMILANI COLLEGE 101 BANKURA CHRISTIAN COLLEGE 103 BANKURA ZILLA SARADAMANI MAHILA MAHAVIDYAPITH 107 CHHATNA CHANDIDAS MAHAVIDYALAYA 120 SONAMUKHI COLLEGE 102 BANKURA SAMMILANI COLLEGE 101 BANKURA CHRISTIAN COLLEGE KABI JAGADRAM ROY GOVERNMENT GENERAL DEGREE 122 COLLEGE, MEJIA 119 SALTORA NETAJI CENTENARY COLLEGE 104 BARJORA COLLEGE 108 GOBINDAPRASAD MAHAVIDYALAYA 110 JAMINI ROY COLLEGE 108 GOBINDA PRASAD MAHAVIDYALAYA 104 BARJORA COLLEGE 110 JAMINI ROY COLLEGE 114 PANDIT RAGHUNATH MURMU SMRITI MAHAVIDYALAYA 113 PANCHMURA MAHAVIDYALAYA 113 PANCHMURA MAHAVIDYALAYA PANDIT RAGHUNATH MURMU SMRITI 114 116 RAIPUR BLOCK MAHAVIDYALAYA MAHAVIDYALAYA 105 BIRSHA MUNDA MEMORIAL COLLEGE 111 KHATRA ADIBASI MAHAVIDYALAYA 118 SALDIHA COLLEGE 123 GOVT. -

Office of the Controller of Examinations
Office of the Controller of Examinations Ref No.: BKU/CE/421/2020 Date: 23/09/2020 NOTIFICATION Sub: Regarding UG SEM VI students with CNC /SNC in UG SEM I SNC Examinations 2019-2020 As directed, it is hereby notified for information of UG SEMESTER VI students with Course Not Cleared (CNC) or Semester Not Cleared (SNC) STATUS in UG SEM I SNC Exams 2019-2020, that Admit Card download Module shall not be available to them right now and can be made available to them in the Unitrack Login Accounts based on their STATUS (whether SQ or SNC) after completion of Post Publication Review of Answer Scripts of UG SEM I SNC Exams 2019-2020, for which they have applied online. The Post Publication Review of Answer Scripts of UG SEM I SNC Exams 2019-2020 of these students is being conducted on an urgent basis, so that their STATUS (whether SQ or SNC) after Post Publication Review can be notified as early as possible. This is as per Bankura University Regulations and Bankura University Examination Regulations. The List of such students is attached. Sd/- Controller of Examinations (Addl. Charge), Bankura University Copy to: 1. The Registrar, Bankura University 2. The Inspector of Colleges, Bankura University 3. All Principals / TiCs / OiCs of affiliated colleges where UG courses are taught 4. The Secretary to the Hon’ble Vice Chancellor, Bankura University 5. Guard File Page 1 of 2 UID Subject Name COLLEGE NAME 17632112005 COMMERCE(H) BANKURA SAMMILANI COLLEGE 17633121006 MATHEMATICS(H) BANKURA SAMMILANI COLLEGE 17331108045 POLITICAL SCIENCE(H) BANKURA -

For Sems I, III & V 2019
Bankura University Routine of End Semester Examinations (Theory) for SEMs I, III & V 2019 - 2020 (11:00 AM to 01:00 PM) ( 02:00 PM to 04:00 PM) Date Day Semester V Semester I (1st Half) Semester V (1st Half) Semester III (2nd Half) (2nd Half) 19/12/2019 THURSDAY Education (P), Chemistry (P) Core 5 (H) Mathematics (P), Commerce DSE1A (P), 20/12/2019 FRIDAY Bengali (P), Botany (P), Commerce 5 (P), Physics (P) Physical Education (P) 21/12/2019 SATURDAY Physiology (P), English (P), Santali (P) Core 6 (H), Sociology (P), Defence Studies (P) 03/01/2020 FRIDAY Political Science (P), Environmental Science (P) Zoology (P), Sanskrit (P), Economics (P) 04/01/2020 SATURDAY Zoology (P), Sanskrit (P), Economics (P) Core 7 (H), History (P) Bengali (P), Botany (P), Commerce DSE 2A (P), 06/01/2020 MONDAY SEC 1 (P) Physics (P) 07/01/2020 TUESDAY DSE 1 (H), Geography (P) Sociology (P) 08/01/2020 WEDNESDAY AECC 1 (H) [ENVS] MIL 2 Core (P) - (Arts & Commerce) 09/01/2020 THURSDAY AECC 1 (P) [ENVS] SEC 1 (H), Philosophy (P), Computer Science (P) 10/01/2020 FRIDAY Mathematics (P), Commerce 2 (P), Physical Education (P) Core 11 (H) Education (P), Chemistry (P) GE 3 (H), Mathematics (P), Commerce 6 (P), 11/01/2020 SATURDAY Physiology (P), English (P) Physical Education (P) 13/01/2020 MONDAY Philosophy (P), Computer Science (P) Core 12 (H) Physiology (P), English (P), Santali (P) 14/01/2020 TUESDAY GE 1 (H),Geography (P), Music (P) Computer Science (P) Political Science (P), Environmental Science (P) 15/01/2020 WEDNESDAY Political Science (P), Environmental Science -
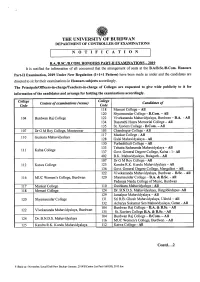
BA, B.Sc. & B.Com
THE UNIVERSITY OF BURDWAN DEPARTMENT OF CONTROLLER OF EXAMINATIONS NOTIFICATION B.A.IB.SC.IB.COM. HONOURS PART-II EXAMINATIONS - 2019 It is notified for information of all concerned that the arrangement of seats at the B.AIB.Sc.IB.Com.Honours Part-II Examination, 2019 Under New Regulation (1+1+1 Pattern) have been made as under and the candidates are directed to sit for their examinations in Honours subjects accordingly. The Principals/Officers-in-chargerreachers-in-charge of Colleges are requested to give wide publicity to it for information of the candidates and arrange for holding the examinations accordingly. College College Centres of examinations (venue) Candidates of Code Code 118 Memari College - All 120 Shyamsundar College - B.Com. - All 104 Burdwan Raj College 122 Vivekananda Mahavidyalaya, Burdwan - B.A. - All 134 Dasarathi Hazra Memorial College - All 135 St. Xaviers College - B.Com. - All 107 Dr G M Roy College, Monteswar 105 Chandrapur College - All 117 Mankar College - All Guskara Mahavidyalaya 110 128 Galsi Mahavidyalaya - All 130 Purbashthali College - All 133 Tehatta Sadananda Mahavidyalaya - All Kalna College 111 137 Govt. General Degree College, Kalna - 1-All 402 B.K. Mahavidyalaya, Balagarh - All 107 Dr G M Roy College - All 112 Katwa College 125 Kandra R.K. Kundu Mahavidyalaya - All 136 Govt. General Degree College, Mangalkot - All 122 Vivekananda Mahavidyalaya, Burdwan - B.Sc. - All 116 MUC Women's College, Burdwan 120 Shyamsundar College - B.A. & B.Sc. - All Padmaja Naidu College of Music, Burdwan 117 Mankar College 110 Gushkara Mahavidyalaya - All 118 Memari College 124 Dr. B.N.D.S. -
Ffhe UNIVERSITY of BURDWAN Depalrtment of CONTROLLER of EXAMINATIONS I INOTIFICATION
ffHE UNIVERSITY OF BURDWAN DEPAlRTMENT OF CONTROLLER OF EXAMINATIONS I INOTIFICATION B.A.IB.St.!B.COM. HONOURS PART-I EXAMINATIONS - 2019 It is notified for information of all concerned that the arrangement of seats at the B.AIB.Sc.IB.Com.Honours Part-I Examination, 2019 underl New Regulation (1+1+1 Pattern) have been made as under and the candidates are directedto sit for their examinationr in Honours subjects accordingly. The Principals/Officers-in-chargrffeachers-in-charge of Colleges are requested to give wide publicity to it for information of the candidates an1 arrange for holding the examinations accordingly. College Centres of examinations College Candidates of Code (venue)1 Code 118 Memari College- - All I 122 Vivekananda Mahavidyalaya, Burdwan - All 104 Burdwan Raj College 124 Dr. B.N.Dutta Smriti Mahavidyalaya, Hatgobindapur- All 134 Dasarathi Hazra Memorial College-All 135 St. Xavier's College- All 112 Katwa College - All 130 Purbasthali College- All 111 Kalna College 133 Tehatta Sadananda Mahavidyalaya- All I, 137 Govt General Degree College, Kalna- All 402 B.K. Mahaviyalaya, Balagarh, Hooghly- All 105 Chandrapur College-All II 107 Dr. G.M. Roy College, Monteswar-All 112 Katwa College 111 Kalna College-All 125 Kandra R. K. Kundu Mahavidyalaya-All I: 136 Govt. General Degree College, Mongalkote-All 1'29 Jamalpur Mahavidyalaya- All 120 Shyamsundar College 131 Sir R.B.Ghosh Mahavidyalaya, Ukhrid- All 132 Acharya Sukumar Sen Mahavidyalaya, Gotan- All 104 Burdwan Raj College - All 110 Vivekananda Mahavidyalaya, Guskara Mahavidyalaya-All 122 116 MUC Women's college- All Burdwan I 120 Shyamsundar College- - All 128 Galsi Mahavidyalaya- All 201 Bankura Christian College - All I 205 G.P. -
Economics Total General Sc St Obc(A) Obc(B) Ph/Vh Vacancy 22 79 32 11 12 2 158
ECONOMICS TOTAL GENERAL SC ST OBC(A) OBC(B) PH/VH VACANCY 22 79 32 11 12 2 158 GENERAL University Sl No. College Total 1 DESHBANDHU MAHAVIDYALAYA 1 2 DURGAPUR WOMEN'S COLLEGE 1 3 KANDRA RADHA KANTO KUNDU MAHAVIDYALAYA 1 BURDWAN UNIVERSITY 4 PANCHMURA MAHAVIDYALAYA 1 5 SALDIHA COLLEGE 1 6 SAMBHUNATH COLLEGE 1 7 T.D.B. COLLEGE 1 8 ASUTOSH COLLEGE 1 9 K.K.DAS COLLEGE 1 10 NARASINHA DUTT COLLEGE 1 CALCUTTA UNIVERSITY 11 PRABHU JAGATBANDHU COLLEGE 1 12 SETH ANANDARAM JAIPURIA COLLEGE 1 13 SUNDARBAN HAZI DESARAT COLLEGE 1 GOURBANGA UNIVERSITY 14 BALURGHAT COLLEGE 1 15 BERHAMPUR COLLEGE 1 KALYANI UNIVERSITY 16 KANCHRAPARA COLLEGE 1 17 SUDHIRRANJAN LAHIRI MAHAVIDYALAYA 1 18 BHATTER COLLEGE 1 VIDYASAGAR UNIVERSITY 19 MIDNAPUR COLLEGE 1 20 BHAIRAB GANGULY COLLEGE 1 WEST BENGAL STATE UNIVERSITY 21 GOBARDANGA HINDU COLLEGE 1 22 NAHATA JOGENDRANATH MONDAL SMRITI MAHAVIDYALAYA 1 OBC(A) 1 BANKURA SAMMILANI COLLEGE 1 BURDWAN UNIVERSITY 2 DESHBANDHU MAHAVIDYALAYA 1 3 RABINDRA MV 1 4 CITY COLLEGE 1 5 CITY COLLEGE OF COMMERCE & BUSINESS ADMINISTRATION 1 CALCUTTA UNIVERSITY 6 HERAMBA CHANDRA COLLEGE 1 7 RAMSADAY COLLEGE 1 KALYANI UNIVERSITY 8 RANI DHANYA KUMARI COLLEGE 1 SIDHO KANHO BIRSA UNIVERSITY 9 NISTARINI COLLEGE 1 10 BASIRHAT COLLEGE 1 WEST BENGAL STATE UNIVERSITY 11 HIRALAL MAZUMDAR MEMORIAL COLLEGE FOR WOMEN 1 OBC(B) BURDWAN UNIVERSITY 1 KATWA COLLEGE 1 2 ANANDA MOHAN COLLEGE 1 3 CALCUTTA GIRLS' COLLEGE 1 CALCUTTA UNIVERSITY 4 JOGMAYA DEVI COLLEGE 1 5 MAHESHTALA COLLEGE 1 6 BALURGHAT COLLEGE 1 GOURBANGA UNIVERSITY 7 RAIGANJ SURENDRANATH MAHAVIDYALAYA