John Joly (1857–1933)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Martian Crater Morphology
ANALYSIS OF THE DEPTH-DIAMETER RELATIONSHIP OF MARTIAN CRATERS A Capstone Experience Thesis Presented by Jared Howenstine Completion Date: May 2006 Approved By: Professor M. Darby Dyar, Astronomy Professor Christopher Condit, Geology Professor Judith Young, Astronomy Abstract Title: Analysis of the Depth-Diameter Relationship of Martian Craters Author: Jared Howenstine, Astronomy Approved By: Judith Young, Astronomy Approved By: M. Darby Dyar, Astronomy Approved By: Christopher Condit, Geology CE Type: Departmental Honors Project Using a gridded version of maritan topography with the computer program Gridview, this project studied the depth-diameter relationship of martian impact craters. The work encompasses 361 profiles of impacts with diameters larger than 15 kilometers and is a continuation of work that was started at the Lunar and Planetary Institute in Houston, Texas under the guidance of Dr. Walter S. Keifer. Using the most ‘pristine,’ or deepest craters in the data a depth-diameter relationship was determined: d = 0.610D 0.327 , where d is the depth of the crater and D is the diameter of the crater, both in kilometers. This relationship can then be used to estimate the theoretical depth of any impact radius, and therefore can be used to estimate the pristine shape of the crater. With a depth-diameter ratio for a particular crater, the measured depth can then be compared to this theoretical value and an estimate of the amount of material within the crater, or fill, can then be calculated. The data includes 140 named impact craters, 3 basins, and 218 other impacts. The named data encompasses all named impact structures of greater than 100 kilometers in diameter. -

Widespread Crater-Related Pitted Materials on Mars: Further Evidence for the Role of Target Volatiles During the Impact Process ⇑ Livio L
Icarus 220 (2012) 348–368 Contents lists available at SciVerse ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus Widespread crater-related pitted materials on Mars: Further evidence for the role of target volatiles during the impact process ⇑ Livio L. Tornabene a, , Gordon R. Osinski a, Alfred S. McEwen b, Joseph M. Boyce c, Veronica J. Bray b, Christy M. Caudill b, John A. Grant d, Christopher W. Hamilton e, Sarah Mattson b, Peter J. Mouginis-Mark c a University of Western Ontario, Centre for Planetary Science and Exploration, Earth Sciences, London, ON, Canada N6A 5B7 b University of Arizona, Lunar and Planetary Lab, Tucson, AZ 85721-0092, USA c University of Hawai’i, Hawai’i Institute of Geophysics and Planetology, Ma¯noa, HI 96822, USA d Smithsonian Institution, Center for Earth and Planetary Studies, Washington, DC 20013-7012, USA e NASA Goddard Space Flight Center, Greenbelt, MD 20771, USA article info abstract Article history: Recently acquired high-resolution images of martian impact craters provide further evidence for the Received 28 August 2011 interaction between subsurface volatiles and the impact cratering process. A densely pitted crater-related Revised 29 April 2012 unit has been identified in images of 204 craters from the Mars Reconnaissance Orbiter. This sample of Accepted 9 May 2012 craters are nearly equally distributed between the two hemispheres, spanning from 53°Sto62°N latitude. Available online 24 May 2012 They range in diameter from 1 to 150 km, and are found at elevations between À5.5 to +5.2 km relative to the martian datum. The pits are polygonal to quasi-circular depressions that often occur in dense clus- Keywords: ters and range in size from 10 m to as large as 3 km. -

Mars Science Laboratory: Curiosity Rover Curiosity’S Mission: Was Mars Ever Habitable? Acquires Rock, Soil, and Air Samples for Onboard Analysis
National Aeronautics and Space Administration Mars Science Laboratory: Curiosity Rover www.nasa.gov Curiosity’s Mission: Was Mars Ever Habitable? acquires rock, soil, and air samples for onboard analysis. Quick Facts Curiosity is about the size of a small car and about as Part of NASA’s Mars Science Laboratory mission, Launch — Nov. 26, 2011 from Cape Canaveral, tall as a basketball player. Its large size allows the rover Curiosity is the largest and most capable rover ever Florida, on an Atlas V-541 to carry an advanced kit of 10 science instruments. sent to Mars. Curiosity’s mission is to answer the Arrival — Aug. 6, 2012 (UTC) Among Curiosity’s tools are 17 cameras, a laser to question: did Mars ever have the right environmental Prime Mission — One Mars year, or about 687 Earth zap rocks, and a drill to collect rock samples. These all conditions to support small life forms called microbes? days (~98 weeks) help in the hunt for special rocks that formed in water Taking the next steps to understand Mars as a possible and/or have signs of organics. The rover also has Main Objectives place for life, Curiosity builds on an earlier “follow the three communications antennas. • Search for organics and determine if this area of Mars was water” strategy that guided Mars missions in NASA’s ever habitable for microbial life Mars Exploration Program. Besides looking for signs of • Characterize the chemical and mineral composition of Ultra-High-Frequency wet climate conditions and for rocks and minerals that ChemCam Antenna rocks and soil formed in water, Curiosity also seeks signs of carbon- Mastcam MMRTG • Study the role of water and changes in the Martian climate over time based molecules called organics. -
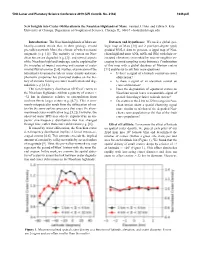
New Insights Into Crater Obliteration in the Noachian Highlands of Mars
50th Lunar and Planetary Science Conference 2019 (LPI Contrib. No. 2132) 1649.pdf New Insights into Crater Obliteration in the Noachian Highlands of Mars. Samuel J. Holo and Edwin S. Kite University of Chicago, Department of Geophysical Sciences, Chicago, IL, 60637 – [email protected] Introduction: The Noachian highlands of Mars are Datasets and hypotheses: We used a global geo- heavily-cratered terrain that, in their geology, record logic map of Mars [10] and 2 pixel-per-degree (ppd) pre-valley-network Mars, the climate of which remains gridded MOLA data to generate a 2ppd map of Noa- enigmatic (e.g. [1]). The majority of craters on Noa- chian highland units (eNh, mNh and lNh) with their as- chian terrain are degraded (e.g. [2]), and several aspects sociated elevations (smoothed by nearest-neighbor av- of the Noachian highland landscape can be explained by eraging to avoid sampling crater bottoms). Combination the interplay of impact cratering and erosion of crater of this map with a global database of Martian craters rims by fluvial erosion [3,4]. Further, examination of the [11] enables us to ask four main questions: latitudinal/elevational trends in crater density and mor- • Is there a signal of a latitude control on crater phometric properties has prompted studies on the his- obliteration? tory of climatic forcing on crater modification and deg- • Is there a signal of an elevation control on radation (e.g. [2,5]). crater obliteration? The size-frequency distribution (SFD) of craters in • Does the degradation of equatorial craters on the Noachian highlands exhibits a paucity of craters < Noachian terrain leave a measurable signal of ~32 km in diameter, relative to extrapolation from spatial clustering relative to fresh craters? isochron fits to larger craters (e.g. -

Ancient Fluid Escape and Related Features in Equatorial Arabia Terra (Mars)
EPSC Abstracts Vol. 7 EPSC2012-132-3 2012 European Planetary Science Congress 2012 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2012 Ancient fluid escape and related features in equatorial Arabia Terra (Mars) F. Franchi(1), A. P. Rossi(2), M. Pondrelli(3), B. Cavalazzi(1), R. Barbieri(1), Dipartimento di Scienze della Terra e Geologico Ambientali, Università di Bologna, via Zamboni 67, 40129 Bologna, Italy ([email protected]). 2Jacobs University, Bremen, Germany. 3IRSPS, Università D’Annunzio, Pescara, Italy. Abstract Noachian [1]. The ELDs are composed by light rocks showing a polygonal pattern, described elsewhere on Arabia Terra, in the equatorial region of Mars, is Mars [4], and is characterized by a high sinuosity of long-time studied area especially for the abundance the strata that locally follows a concentric trend of fluid related features. Detailed stratigraphic and informally called “pool and rim” structures (Fig. morphological study of the succession exposed in the 1A). Crommelin and Firsoff craters evidenced the occurrences of flow structures and spring deposits that endorse the presence of fluids circulation in the Late Noachian. All the morphologies in these two proto-basins occur within the Equatorial Layered Deposits (ELDs). 1. Introduction Martian layered spring deposits are of considerable interest for their supposed relationship with water and high potential of microbial signatures preservation. Their supposed fluid-related origin [1] makes the Equatorial Layered Deposits attractive targets for future missions with astrobiological purposes. In this study we report the occurrence of mounds fields and flow structures in Firsoff and Crommelin craters and summarize the result of a detailed study of the remote-sensing data sets available in this region. -

FROM WET PLANET to RED PLANET Current and Future Exploration Is Shaping Our Understanding of How the Climate of Mars Changed
FROM WET PLANET TO RED PLANET Current and future exploration is shaping our understanding of how the climate of Mars changed. Joel Davis deciphers the planet’s ancient, drying climate 14 DECEMBER 2020 | WWW.GEOLSOC.ORG.UK/GEOSCIENTIST WWW.GEOLSOC.ORG.UK/GEOSCIENTIST | DECEMBER 2020 | 15 FEATURE GEOSCIENTIST t has been an exciting year for Mars exploration. 2020 saw three spacecraft launches to the Red Planet, each by diff erent space agencies—NASA, the Chinese INational Space Administration, and the United Arab Emirates (UAE) Space Agency. NASA’s latest rover, Perseverance, is the fi rst step in a decade-long campaign for the eventual return of samples from Mars, which has the potential to truly transform our understanding of the still scientifi cally elusive Red Planet. On this side of the Atlantic, UK, European and Russian scientists are also getting ready for the launch of the European Space Agency (ESA) and Roscosmos Rosalind Franklin rover mission in 2022. The last 20 years have been a golden era for Mars exploration, with ever increasing amounts of data being returned from a variety of landed and orbital spacecraft. Such data help planetary geologists like me to unravel the complicated yet fascinating history of our celestial neighbour. As planetary geologists, we can apply our understanding of Earth to decipher the geological history of Mars, which is key to guiding future exploration. But why is planetary exploration so focused on Mars in particular? Until recently, the mantra of Mars exploration has been to follow the water, which has played an important role in shaping the surface of Mars. -

Appendix I Lunar and Martian Nomenclature
APPENDIX I LUNAR AND MARTIAN NOMENCLATURE LUNAR AND MARTIAN NOMENCLATURE A large number of names of craters and other features on the Moon and Mars, were accepted by the IAU General Assemblies X (Moscow, 1958), XI (Berkeley, 1961), XII (Hamburg, 1964), XIV (Brighton, 1970), and XV (Sydney, 1973). The names were suggested by the appropriate IAU Commissions (16 and 17). In particular the Lunar names accepted at the XIVth and XVth General Assemblies were recommended by the 'Working Group on Lunar Nomenclature' under the Chairmanship of Dr D. H. Menzel. The Martian names were suggested by the 'Working Group on Martian Nomenclature' under the Chairmanship of Dr G. de Vaucouleurs. At the XVth General Assembly a new 'Working Group on Planetary System Nomenclature' was formed (Chairman: Dr P. M. Millman) comprising various Task Groups, one for each particular subject. For further references see: [AU Trans. X, 259-263, 1960; XIB, 236-238, 1962; Xlffi, 203-204, 1966; xnffi, 99-105, 1968; XIVB, 63, 129, 139, 1971; Space Sci. Rev. 12, 136-186, 1971. Because at the recent General Assemblies some small changes, or corrections, were made, the complete list of Lunar and Martian Topographic Features is published here. Table 1 Lunar Craters Abbe 58S,174E Balboa 19N,83W Abbot 6N,55E Baldet 54S, 151W Abel 34S,85E Balmer 20S,70E Abul Wafa 2N,ll7E Banachiewicz 5N,80E Adams 32S,69E Banting 26N,16E Aitken 17S,173E Barbier 248, 158E AI-Biruni 18N,93E Barnard 30S,86E Alden 24S, lllE Barringer 29S,151W Aldrin I.4N,22.1E Bartels 24N,90W Alekhin 68S,131W Becquerei -

Publications
PUBLICATIONS Journal of Geophysical Research: Planets RESEARCH ARTICLE Can perchlorates be transformed to hydrogen peroxide (H2O2) 10.1002/2017JE005329 products by cosmic rays on the Martian surface? Key Points: Parker B. Crandall1,2 , Sándor Góbi1,2, Jeffrey Gillis-Davis3, and Ralf I. Kaiser1,2 • Magnesium perchlorate samples were irradiated with deuterium ions and 1Department of Chemistry, University of Hawai’iatMānoa, Honolulu, Hawaii, USA, 2W.M. Keck Laboratory in Astrochemistry, electrons to model galactic cosmic ray ’ ā 3 ’ (GCR) exposure University of Hawai iatM noa, Honolulu, Hawaii, USA, Hawai i Institute of Geophysics and Planetology, University of • GCRs supplanted in the regolith can Hawai’iatMānoa, Honolulu, Hawaii, USA participate in the formation of hydrogen peroxide via the destruction À of perchlorates Abstract Due to their oxidizing properties, perchlorates (ClO4 ) are suggested by the planetary science • Volatile oxidizing agents formed likely community to play a vital role in the scarcity of organics on the Martian surface. However, alternative contribute to the lack of organic material beneath the Martian surface oxidation agents such as hydrogen peroxide (H2O2) have received surprisingly little attention. In this study, samples of magnesium perchlorate hexahydrate (Mg(ClO4)2 ·6H2O) were exposed to monoenergetic + electrons and D2 ions separately, sequentially, and simultaneously to probe the effects of galactic cosmic ray Correspondence to: exposure of perchlorates and the potential incorporation of hydrogen (deuterium) into these minerals. The R. I. Kaiser, experiments were carried out under ultrahigh-vacuum conditions at 50 K, after which the samples were [email protected] slowly heated to 300 K while the subliming products were monitored by a quadrupole mass spectrometer. -

MARS STUDENT IMAGING PROJECT FINAL REPORT ASU MARS EDUCATION PROGRAM Waubonsie Valley High School | Period 1 | 12‐13 School Year
MARS STUDENT IMAGING PROJECT FINAL REPORT ASU MARS EDUCATION PROGRAM Waubonsie Valley High School | Period 1 | 12‐13 School Year I. Introduction What is your science question? What effect do the polar ice caps have on craters in the rock strata? Why is this question important and interesting? This question is important for the following reasons: 1. Could tell us more about the environmental conditions in the poles and their effects on geologic features. 2. Could tell us which land cover is more likely to preserve signs of past/ present water. List any hypotheses you may have had of what the answer(s) might be to your science question. 1. Craters found on the polar ice caps will be more likely to be destroyed or modified due to the erosional forces of the ice primarily through frost action and basal slip. II. Background Depressions in the This image was collected February 29, 2004 during the end of southern summer season. ice surface caused The local time at the location of the image was about 2 pm. The image shows an area in by sub‐ice craters the South Polar Region. Seasonal changes. The planet's rotation axis is tilted with respect to the orbital plane by almost 24 degrees, so Mars does experience significant seasonal differences in the amount of sunlight falling on a hemisphere during a year. The difference between winter and summer is more extreme on Mars than on Earth, due to the greater eccentricity of the Martian orbit. The Red Planet receives 40 percent more sunlight during its southern summer, when nearest the Sun, than during its southern winter, when the Sun is most distant. -

Lunar and Planetary Science XXXI 1276.Pdf
Lunar and Planetary Science XXXI 1276.pdf THE FATE OF SALT ON MARS : GLOBAL EVIDENCE FOR A NORTHWARD OPEN SALT CYCLE R. D. Lorenz and R. A. Beyer, Lunar and Planetary Laboratory, University of Arizona, Tucson, AZ 85721-0092, USA ([email protected]). Introduction: The general topographic gradient be- ducting a single deposition-dessication cycle, it is more tween the south and north, and the likelihood that likely that the water were cycled through the rocks of oceans on the Martian surface would have been ice- the south several times, leaching out soluble comp o- covered, suggests that the ancient Martian hydrologi- nents each time. Thus the 60m does not indicate an cal cycle had a largely noncycled involatile component upper limit. – salt. It seems likely that massive salt deposits may It is of course likely, especially in such a flood- exist in the low-lying regions of the north and in the ing/dessication cycle, that minerals would be deposited floor of Valles Marineris. in less extensive, but thicker, deposits corresponding Paradigm: Clifford and Parker [1,2] have con- to topographic lows in the surface/groundwater sys- structed a model of Martian hydrology. In particular, tem. they suggest that as the planet cooled, the cryosphere Evidence: Remote sensing evidence exists for would propagate downwards, sealing the aquifer in the evaporite deposits in general (e.g. the ‘White Rock’ [7]) south and freezing over any ocean. The ice-covered and more recently for possible salt domes themselves ocean (for which abundant – albeit largely circumstan- in the floor of Valles Marineris (Beyer et al., this con- tial - evidence now exists [3]) would slowly dry out, the ference) Here we discuss various effects that might be ice being sublimed and deposited on the ice caps. -

Copyrighted Material
1 Geological t ime 1.1 Introduction Why is geological time so important? It underpins everything we study in geology and Earth Science today and provides a framework for many other sciences. The age of the Earth and length of geological time have probably occupied human thought ever since we became conscious of our surround- ings. For centuries, people have attempted to quantify and measure it with varying degrees of success. With a signifi cant degree of certainty, we can now say that the Earth is around 4.65 billion years old, a fi gure that is, to most people, unimaginably long. Many “ creation science ” articles and books that talk about creationist stratigraphy, repeatedly claim that we use and misuse – in their view – geological time. So, is our perception of geology, Earth Science, the rock record, and geological time wrong? Perception of time has changed signifi cantly throughout history, depending on a variety of factors. Some of these will become apparent in the following chapters. When we look at much of the controversy that surrounds geology and other sciences, we fi nd that the perception and determination of time is frequently at the heart of the problem. However, why should this be an issue? The amount of time available for something to occur usually increases the possibility of it happening or the frequency at which it can take place. If time scales are short, changes and variations become more important; but as time scales increase, it is possible for the unusual to become, if not the norm, at least unexceptional. -

Multi-Model Meteorological and Aeolian Predictions for Mars 2020 and the Jezero Crater Region
Space Sci Rev (2021) 217:20 https://doi.org/10.1007/s11214-020-00788-2 Multi-model Meteorological and Aeolian Predictions for Mars 2020 and the Jezero Crater Region C.E. Newman1 · M. de la Torre Juárez2 · J. Pla-García3,4 · R.J. Wilson5 · S.R. Lewis6 · L. Neary7 · M.A. Kahre5 · F. Forget 8 · A. Spiga8,9 · M.I. Richardson1 · F. Daerden7 · T. Bertrand10,5 · D. Viúdez-Moreiras3 · R. Sullivan11 · A. Sánchez-Lavega12 · B. Chide13 · J.A. Rodriguez-Manfredi3 Received: 20 May 2020 / Accepted: 26 December 2020 / Published online: 8 February 2021 © The Author(s) 2021 Abstract Nine simulations are used to predict the meteorology and aeolian activity of the Mars 2020 landing site region. Predicted seasonal variations of pressure and surface and atmospheric temperature generally agree. Minimum and maximum pressure is predicted at Ls ∼ 145◦ and 250◦, respectively. Maximum and minimum surface and atmospheric tem- perature are predicted at Ls ∼ 180◦ and 270◦, respectively; i.e., are warmest at northern fall The Mars 2020 Mission Edited by Kenneth A. Farley, Kenneth H. Williford and Kathryn M. Stack Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11214-020-00788-2. B C.E. Newman [email protected] 1 Aeolis Research, Tucson, AZ, USA 2 Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA 91001, USA 3 Centro de Astrobiología (CSIC-INTA), 28850 Madrid, Spain 4 Space Science Institute, Boulder, CO 80301, USA 5 Ames Research Center, Mountain View, CA, USA 6 The Open