Maine Coastal Observing Alliance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Penobscot Rivershed with Licensed Dischargers and Critical Salmon
0# North West Branch St John T11 R15 WELS T11 R17 WELS T11 R16 WELS T11 R14 WELS T11 R13 WELS T11 R12 WELS T11 R11 WELS T11 R10 WELS T11 R9 WELS T11 R8 WELS Aroostook River Oxbow Smith Farm DamXW St John River T11 R7 WELS Garfield Plt T11 R4 WELS Chapman Ashland Machias River Stream Carry Brook Chemquasabamticook Stream Squa Pan Stream XW Daaquam River XW Whitney Bk Dam Mars Hill Squa Pan Dam Burntland Stream DamXW Westfield Prestile Stream Presque Isle Stream FRESH WAY, INC Allagash River South Branch Machias River Big Ten Twp T10 R16 WELS T10 R15 WELS T10 R14 WELS T10 R13 WELS T10 R12 WELS T10 R11 WELS T10 R10 WELS T10 R9 WELS T10 R8 WELS 0# MARS HILL UTILITY DISTRICT T10 R3 WELS Water District Resevoir Dam T10 R7 WELS T10 R6 WELS Masardis Squapan Twp XW Mars Hill DamXW Mule Brook Penobscot RiverYosungs Lakeh DamXWed0# Southwest Branch St John Blackwater River West Branch Presque Isle Strea Allagash River North Branch Blackwater River East Branch Presque Isle Strea Blaine Churchill Lake DamXW Southwest Branch St John E Twp XW Robinson Dam Prestile Stream S Otter Brook L Saint Croix Stream Cox Patent E with Licensed Dischargers and W Snare Brook T9 R8 WELS 8 T9 R17 WELS T9 R16 WELS T9 R15 WELS T9 R14 WELS 1 T9 R12 WELS T9 R11 WELS T9 R10 WELS T9 R9 WELS Mooseleuk Stream Oxbow Plt R T9 R13 WELS Houlton Brook T9 R7 WELS Aroostook River T9 R4 WELS T9 R3 WELS 9 Chandler Stream Bridgewater T T9 R5 WELS TD R2 WELS Baker Branch Critical UmScolcus Stream lmon Habitat Overlay South Branch Russell Brook Aikens Brook West Branch Umcolcus Steam LaPomkeag Stream West Branch Umcolcus Stream Tie Camp Brook Soper Brook Beaver Brook Munsungan Stream S L T8 R18 WELS T8 R17 WELS T8 R16 WELS T8 R15 WELS T8 R14 WELS Eagle Lake Twp T8 R10 WELS East Branch Howe Brook E Soper Mountain Twp T8 R11 WELS T8 R9 WELS T8 R8 WELS Bloody Brook Saint Croix Stream North Branch Meduxnekeag River W 9 Turner Brook Allagash Stream Millinocket Stream T8 R7 WELS T8 R6 WELS T8 R5 WELS Saint Croix Twp T8 R3 WELS 1 Monticello R Desolation Brook 8 St Francis Brook TC R2 WELS MONTICELLO HOUSING CORP. -

Saco River Saco & Biddeford, Maine
Environmental Assessment Finding of No Significant Impact, and Section 404(b)(1) Evaluation for Maintenance Dredging DRAFT Saco River Saco & Biddeford, Maine US ARMY CORPS OF ENGINEERS New England District March 2016 Draft Environmental Assessment: Saco River FNP DRAFT ENVIRONMENTAL ASSESSMENT FINDING OF NO SIGNIFICANT IMPACT Section 404(b)(1) Evaluation Saco River Saco & Biddeford, Maine FEDERAL NAVIGATION PROJECT MAINTENANCE DREDGING March 2016 New England District U.S. Army Corps of Engineers 696 Virginia Rd Concord, Massachusetts 01742-2751 Table of Contents 1.0 INTRODUCTION ........................................................................................... 1 2.0 PROJECT HISTORY, NEED, AND AUTHORITY .......................................... 1 3.0 PROPOSED PROJECT DESCRIPTION ....................................................... 3 4.0 ALTERNATIVES ............................................................................................ 6 4.1 No Action Alternative ..................................................................................... 6 4.2 Maintaining Channel at Authorized Dimensions............................................. 6 4.3 Alternative Dredging Methods ........................................................................ 6 4.3.1 Hydraulic Cutterhead Dredge....................................................................... 7 4.3.2 Hopper Dredge ........................................................................................... 7 4.3.3 Mechanical Dredge .................................................................................... -

Comprehensive Plan Vol. 1, Part 4
Vol. I, 2009 Edgecomb Comprehensive Plan 24 PART 4 NATURAL RESOURCES CRITICAL NATURAL RESOURCES MAINE’S GROWTH MANAGEMENT GOAL To protect the state's other critical natural resources, including without limitation, wetlands, wildlife and fisheries habitat, sand dunes, shorelands, scenic vistas, and unique natural areas. TOWN VISION To protect Edgecomb’s critical natural resources within and surrounding Edgecomb’s privately- owned undeveloped and unfragmented lands; Edgecomb’s only great pond, Lily Pond; the town- owned Charles and Constance Schmid Land Preserve as well as Edgecomb’s tidal frontage and its scenic vistas. CITIZENS’ VIEW (SURVEY RESPONSE) ● 58%, or 205 respondents, choose to live in Edgecomb because of its proximity to water, clear skies and starry nights. ● 54%, or 177 respondents, enjoy the respect for privacy in Edgecomb. Unfragmented Parcels ● 71%, or 253 respondents, defined rural as (Source: Beginning with Habitat) “the bulk of our land remaining undeveloped, with large tracts of backland, fields and forests.” ● 28%, or 94 respondents, objected to forestry operations “in their back yard.” ● 54%, or 191 respondents, felt that nature preserves are an acceptable trade-off for lost tax revenue. CONDITIONS AND TRENDS The topography of the upper part of the peninsula comprising the Town of Edgecomb is typical of Maine coastline peninsulas. A gently rolling landscape of rocky, clay soil, remaining from land which was heavily wooded before clearing and settlement of the 18th century, is laid over a granite skeleton. A mixture of second and third growth woodland is broken by the pattern of open fields surviving from 18th and 19th century farms when agriculture and fishing were the major sources of livelihood for inhabitants. -

Comparison of Observed and Predicted Abutment Scour at Selected Bridges in Maine
Comparison of Observed and Predicted Abutment Scour at Selected Bridges in Maine By Pamela J. Lombard and Glenn A. Hodgkins Prepared in cooperation with the Maine Department of Transportation Scientific Investigations Report 2008–5099 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark D. Myers, Director U.S. Geological Survey, Reston, Virginia: 2008 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov Telephone: 1-888-ASK-USGS Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Lombard, P.J., and Hodgkins, G.A., 2008, Comparison of observed and predicted abutment scour at selected bridges in Maine: U.S. Geological Survey Scientific Investigations Report 2008–5099, 23 p., available only online at http://pubs.usgs.gov/sir/2008/5099. iii Contents Abstract ...........................................................................................................................................................1 Introduction.....................................................................................................................................................1 -

Lady Crabs, Ovalipes Ocellatus, in the Gulf of Maine
18_04049_CRABnotes.qxd 6/5/07 8:16 PM Page 106 Notes Lady Crabs, Ovalipes ocellatus, in the Gulf of Maine J. C. A. BURCHSTED1 and FRED BURCHSTED2 1 Department of Biology, Salem State College, Salem, Massachusetts 01970 USA 2 Research Services, Widener Library, Harvard University, Cambridge, Massachusetts 02138 USA Burchsted, J. C. A., and Fred Burchsted. 2006. Lady Crabs, Ovalipes ocellatus, in the Gulf of Maine. Canadian Field-Naturalist 120(1): 106-108. The Lady Crab (Ovalipes ocellatus), mainly found south of Cape Cod and in the southern Gulf of St. Lawrence, is reported from an ocean beach on the north shore of Massachusetts Bay (42°28'60"N, 70°46'20"W) in the Gulf of Maine. All previ- ously known Gulf of Maine populations north of Cape Cod Bay are estuarine and thought to be relicts of a continuous range during the Hypsithermal. The population reported here is likely a recent local habitat expansion. Key Words: Lady Crab, Ovalipes ocellatus, Gulf of Maine, distribution. The Lady Crab (Ovalipes ocellatus) is a common flats (Larsen and Doggett 1991). Lady Crabs were member of the sand beach fauna south of Cape Cod. not found in intensive local studies of western Cape Like many other members of the Virginian faunal Cod Bay (Davis and McGrath 1984) or Ipswich Bay province (between Cape Cod and Cape Hatteras), it (Dexter 1944). has a disjunct population in the southern Gulf of St. Berrick (1986) reports Lady Crabs as common on Lawrence (Ganong 1890). The Lady Crab is of consid- Cape Cod Bay sand flats (which commonly reach 20°C erable ecological importance as a consumer of mac- in summer). -

Casco Bay Weekly : 13 July 1989
Portland Public Library Portland Public Library Digital Commons Casco Bay Weekly (1989) Casco Bay Weekly 7-13-1989 Casco Bay Weekly : 13 July 1989 Follow this and additional works at: http://digitalcommons.portlandlibrary.com/cbw_1989 Recommended Citation "Casco Bay Weekly : 13 July 1989" (1989). Casco Bay Weekly (1989). 28. http://digitalcommons.portlandlibrary.com/cbw_1989/28 This Newspaper is brought to you for free and open access by the Casco Bay Weekly at Portland Public Library Digital Commons. It has been accepted for inclusion in Casco Bay Weekly (1989) by an authorized administrator of Portland Public Library Digital Commons. For more information, please contact [email protected]. Greater Portland's news and arts weekly JULY 13, 1989 FREE ... that don't make THE NEWS (OYER STOll by Kelly Nelson PHOTOS by Tonet! Harbert One night last April Michael Metevier got off work at midnight and headed over to Raoul's to hear some blues. An hour later he was cruising home, feeling good. His tune changed when he got home. His door was smashed open. The lock lay useless on the floor. The lights were -. glaring. "It was quite a bunch of mixed emotions - shock and being violated. I was kind of in a daze," says Metevier of finding his home burglarized. He didn't sleep well that night. He kept thinking that someone he didn't know had been in his home - and had stolen his telephone, answering machine, flashlight, calculator, candy dish, towel!! and electric shaver. You probably heard every gory detail of the four murders in the Portland area last year. -

Narraguagus River Water Quality Monitoring Plan
Narraguagus River Water Quality Monitoring Plan A Guide for Coordinated Water Quality Monitoring Efforts in an Atlantic Salmon Watershed in Maine By Barbara S. Arter BSA Environmental Consulting And Barbara Snapp, Ph. D. January 2006 Sponsored By The Narraguagus River Watershed Council Funded By The National Fish and Wildlife Foundation Narraguagus River Water Quality Monitoring Plan A Guide for Coordinated Water Quality Monitoring Efforts in an Atlantic Salmon Watershed in Maine By Barbara S. Arter BSA Environmental Consulting And Barbara Snapp, Ph. D. January 2006 Sponsored By The Narraguagus River Watershed Council Funded By The National Fish and Wildlife Foundation Narraguagus River Water Quality Monitoring Plan Preface In an effort to enhance water quality monitoring (WQM) coordination among agencies and conservation organizations, the Project SHARE Research and Management Committee initiated a program whereby river-specific WQM Plans are developed for Maine rivers that currently contain Atlantic salmon populations listed in the Endangered Species Act. The Sheepscot River WQM Plan was the first plan to be developed under this initiative. It was developed between May 2003 and June 2004. The Action Items were finalized and the document signed in March 2005 (Arter, 2005). The Narraguagus River WQM Plan is the second such plan and was produced by a workgroup comprised of representatives from both state and federal government agencies and several conservation organizations (see Acknowledgments). The purpose of this plan is to characterize current WQM activities, describe current water quality trends, identify the role of each monitoring agency, and make recommendations for future monitoring. The project was funded by the National Fish and Wildlife Foundation. -

1982 Maine River's Study Appendix H - Rivers with Historical Landmarks & Register Sites
1982 Maine River's Study Appendix H - Rivers with Historical Landmarks & Register Sites HISTORI RIVER NAME HISTORIC SITE/PLACE C COUNTY LOCATION LINK Androscoggin River Pejepscot Paper Mill RHP Sagadahoc Topsham https://www.mainememory.net/sitebuilder/site/201/page/460/display Androscoggin River Barker Mill RHP Androscoggin Auburn https://tinyurl.com/y8wsy2a6 Bagaduce River Fort George RHP Hancock Castine https://en.wikipedia.org/wiki/Fort_George_(Castine,_Maine) Carrabasset River (Lemon Stream) New Portland Wire Bridge RHP Somerset New Portland http://www.maine.gov/mdot/historicbridges/otherbridges/wirebridge/index.shtml Damariscotta Oyster Shell Heaps (Whaleback) Damariscotta River RHP Lincoln Damariscotta http://tinyurl.com/m9vgk84 Kennebec Franklin Dead River Dead River Arnold Trail to Quebec RHP Somerset Chain of Ponds http://en.wikipedia.org/wiki/Benedict_Arnold%27s_expedition_to_Quebec Ellis River Lovejoy Bridge RHP Oxford South Andover http://www.maine.gov/mdot/historicbridges/coveredbridges/lovejoybridge/ Kenduskeag Stream Robyville Bridge RHP Penobscot Bangor http://www.maine.gov/mdot/historicbridges/coveredbridges/robyvillebridge/ Kenduskeag Stream Morse Bridge RHP Penobscot Bangor http://bangorinfo.com/Focus/focus_kenduskeag_stream.html Kennebec River Fort Baldwin RHP Sagadahoc Popham Beach http://www.maine.gov/cgi-bin/online/doc/parksearch/details.pl?park_id=86 Kennebec River Fort Popham RHP Sagadahoc Popham Beach http://www.fortwiki.com/Fort_Popham Percy and Small Shipyard Kennebec River Maritime Museum District* RHP Sagadahoc -

Notice to Flood Insurance Study Users
LINCOLN COUNTY, MAINE (ALL JURISDICTIONS) Lincoln County COMMUNITY NAME COMMUNITY NUMBER COMMUNITY NAME COMMUNITY NUMBER Alna, Town of 230083 Monhegan Plantation 230511 Bar Island 230916 Newcastle, Town of 230218 Boothbay, Town of 230212 Nobleboro, Town of 230219 Boothbay Harbor, Town of 230213 Polins Ledges Island 230929 Bremen, Town of 230214 Ross Island 230922 Bristol, Town of 230215 Somerville, Town of 230512 Damariscotta, Town of 230216 South Bristol, Town of 230220 Dresden, Town of 230084 Southport, Town of 230221 Edgecomb, Town of 230217 Thief Island 230920 Haddock Island 230918 Thrumcap Island 230928 Hibberts Gore, Township of 230712 Waldoboro, Town of 230086 Hungry Island 230917 Webber Dry Ledge Island 230930 Indian Island 230919 Western Egg Rock Island 230926 Jefferson, Town of 230085 Westport, Town of 230222 Jones Garden Island 230925 Whitefield, Town of 230087 Killick Stone Island 230927 Wiscasset, Town of 230223 Louds Island 230915 Wreck Island 230924 Marsh Island 230921 Wreck Island Ledge 230923 PRELIMINARY DATE: February 7, 2014 Federal Emergency Management Agency FLOOD INSURANCE STUDY NUMBER 23015CV001A NOTICE TO FLOOD INSURANCE STUDY USERS Communities participating in the National Flood Insurance Program have established repositories of flood hazard data for floodplain management and flood insurance purposes. This Flood Insurance Study (FIS) report may not contain all data available within the Community Map Repository. Please contact the Community Map Repository for any additional data. The Federal Emergency Management Agency (FEMA) may revise and republish part or all of this FIS report at any time. In addition, FEMA may revise part of this FIS report by the Letter of Map Revision process, which does not involve republication or redistribution of the FIS report. -

The Maine Area Were Assigned to the Various Forts
The Friendship Sloop "Pemaquid" in Fiberglass LOA - 25' LWL - 21' Beam - 8' 8" DEDICATION Draft - 4' 2" Your editor would like to take it upon himself to dedicate this year's booklet without consulting the POWERS THAT BE. He's sure you have Disp. - 7000 Ibs. noticed the ever increasing quality of this program as years go by. This Keel - 2000 Ibs. is due to the number of contributors of material who have come forward in late years. Instead of writing 90% of the "stuff you read here, he S.A. - 432' now only has to write 10 percent. So to those of you who lend a helping hand — Many thanks! Keep it up! — Don't quit now! — See you next year! and thanks again! President's Message Some time ago some one said, "The only thing that is permanent is This Sloop sleeps four with Galley, Head, Volvo Diesel, Wheel Steering, change." However change for changes sake alone is wrong. Bronze Hardware, Lignum Vitae Blocks and Deadeyes, All Teak Being a member and participating in the activities of the Friendship Woodwork, Native Spruce Spars, and Dacron Sails. Sloop Society is a wonderful experience. The success of the Society is mostly because of the hard work of those who have done so much to HULL AND DECK MOLDING — JARVIS NEWMAN keep up the interest by constantly making changes that are positive im- Southwest Harbor, Maine — (207) 244-3860 provements in the many facets of the Society's activities. As usual these workers are a small percentage of the total member- COMPLETION AND FINISHING — TOMAS D. -
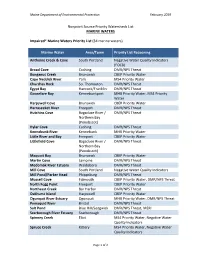
Nonpoint Source Priority Watersheds List MARINE WATERS
Maine Department of Environmental Protection February 2019 Nonpoint Source Priority Watersheds List MARINE WATERS Impaired* Marine Waters Priority List (34 marine waters) Marine Water Area/Town Priority List Reasoning Anthoine Creek & Cove South Portland Negative Water Quality Indicators (FOCB) Broad Cove Cushing DMR/NPS Threat Bunganuc Creek Brunswick CBEP Priority Water Cape Neddick River York MS4 Priority Water Churches Rock So. Thomaston DMR/NPS Threat Egypt Bay Hancock/Franklin DMR/NPS Threat Goosefare Bay Kennebunkport MHB Priority Water, MS4 Priority Water Harpswell Cove Brunswick CBEP Priority Water Harraseeket River Freeport DMR/NPS Threat Hutchins Cove Bagaduce River / DMR/NPS Threat Northern Bay (Penobscot) Hyler Cove Cushing DMR/NPS Threat Kennebunk River Kennebunk MHB Priority Water Little River and Bay Freeport CBEP Priority Water Littlefield Cove Bagaduce River / DMR/NPS Threat Northern Bay (Penobscot) Maquoit Bay Brunswick CBEP Priority Water Martin Cove Lamoine DMR/NPS Threat Medomak River Estuary Waldoboro DMR/NPS Threat Mill Cove South Portland Negative Water Quality Indicators Mill Pond/Parker Head Phippsburg DMR/NPS Threat Mussell Cove Falmouth CBEP Priority Water, DMR/NPS Threat North Fogg Point Freeport CBEP Priority Water Northeast Creek Bar Harbor DMR/NPS Threat Oakhurst Island Harpswell CBEP Priority Water Ogunquit River Estuary Ogunquit MHB Priority Water, DMR/NPS Threat Pemaquid River Bristol DMR/NPS Threat Salt Pond Blue Hill/Sedgwick DMR/NPS Threat, MERI Scarborough River Estuary Scarborough DMR/NPS Threat Spinney Creek Eliot MS4 Priority Water, Negative Water Quality Indicators Spruce Creek Kittery MS4 Priority Water, Negative Water Quality Indicators Page 1 of 2 MDEP NPS Priority Watersheds List – MARINE WATERS February 2019 Marine Water Area/Town Priority List Reasoning Spurwink River Scarborough MHB Priority Water, DMR/NPS Threat St. -

A Technical Characterization of Estuarine and Coastal New Hampshire New Hampshire Estuaries Project
AR-293 University of New Hampshire University of New Hampshire Scholars' Repository PREP Publications Piscataqua Region Estuaries Partnership 2000 A Technical Characterization of Estuarine and Coastal New Hampshire New Hampshire Estuaries Project Stephen H. Jones University of New Hampshire Follow this and additional works at: http://scholars.unh.edu/prep Part of the Marine Biology Commons Recommended Citation New Hampshire Estuaries Project and Jones, Stephen H., "A Technical Characterization of Estuarine and Coastal New Hampshire" (2000). PREP Publications. Paper 294. http://scholars.unh.edu/prep/294 This Report is brought to you for free and open access by the Piscataqua Region Estuaries Partnership at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in PREP Publications by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. A Technical Characterization of Estuarine and Coastal New Hampshire Published by the New Hampshire Estuaries Project Edited by Dr. Stephen H. Jones Jackson estuarine Laboratory, university of New Hampshire Durham, NH 2000 TABLE OF CONTENTS ACKNOWLEDGEMENTS TABLE OF CONTENTS ............................................................................................i LIST OF TABLES ....................................................................................................vi LIST OF FIGURES.................................................................................................viii