Valsartan) Tablets, for Oral Use Diovan in Patients with Diabetes (4) Initial U.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Effective Dose Range of Enalapril in Mild to Moderate Essential Hypertension
Br. J. clin. Pharmac. (1985), 19, 605-611 Effective dose range of enalapril in mild to moderate essential hypertension R. BERGSTRAND', H. HERLITZ2, SAGA JOHANSSON', G. BERGLUND2, A. VEDIN', C. WILHELMSSON', H. J. GOMEZ3, V. J. CIRILLO3 & J. A. BOLOGNESE4 'Department of Medicine, Ostra Hospital and 2Department of Medicine I, Sahlgrenska Hospital, Goteborg, Sweden and Department of 3Cardiovascular Clinical Research and 4Clinical Biostatistics, Merck Sharp & Dohme Research Laboratories, Rahway, New Jersey, USA 1 The dose-response relationship of enalapril was evaluated in a double-blind, balanced, two-period, incomplete-block study in 91 patients with mild to moderate essential hyper- tension. 2 Patients were randomly assigned to two of six treatments: placebo, 2.5, 5, 10, 20 and 40 mg/day of enalapril maleate. There were two 3-week treatment periods, each preceded by a 4-week, single-blind placebo washout. 3 Each dose of enalapril produced significant decreases in standing and supine systolic and diastolic blood pressure after 2 and 3 weeks of treatment. There were no significant changes on placebo. 4 There was a significant linear dose response relationship for both mean blood pressure and mean change from baseline in blood pressure (P < 0.01 for systolic and mean arterial pressure, and P < 0.05 for diastolic pressure). 5 Enalapril was associated with an increasing dose-response relationship across the 2.5- 40 mg/day range. The 2.5 mg/dose is effective in some patients; however, doses ¢ 10 mg/ day may be necessary to achieve satisfactory blood pressure control. Keywords enalapril angiotensin converting enzyme inhibitor dose-response relationship Introduction In recent years much interest has been focused with renal impairment treated with high doses of on angiotensin converting enzyme (ACE) in- captopril. -

Diovan® Valsartan
NDA 21-283/S-011 Page 3 T2005-02 Diovan® valsartan Tablets Rx only Prescribing Information USE IN PREGNANCY When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, Diovan should be discontinued as soon as possible. See WARNINGS: Fetal/Neonatal Morbidity and Mortality. DESCRIPTION Diovan® (valsartan) is a nonpeptide, orally active, and specific angiotensin II antagonist acting on the AT1 receptor subtype. Valsartan is chemically described as N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl) [1,1′-biphenyl]- 4-yl]methyl]-L-valine. Its empirical formula is C24H29N5O3, its molecular weight is 435.5, and its structural formula is Valsartan is a white to practically white fine powder. It is soluble in ethanol and methanol and slightly soluble in water. NDA 21-283/S-011 Page 4 Diovan is available as tablets for oral administration, containing 40 mg, 80 mg, 160 mg or 320 mg of valsartan. The inactive ingredients of the tablets are colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, iron oxides (yellow, black and/or red), magnesium stearate, microcrystalline cellulose, polyethylene glycol 8000, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Valsartan blocks the vasoconstrictor and aldosterone- secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. -
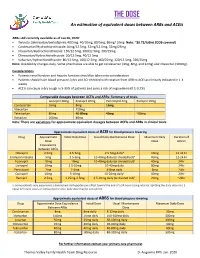
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Diovan (Valsartan) Tablets ------DOSAGE FORMS and STRENGTHS------Initial U.S
HIGHLIGHTS OF PRESCRIBING INFORMATION without food. In heart failure patients, consideration should be given to These highlights do not include all the information needed to use Diovan reducing the dose of concomitant diuretics. Following myocardial safely and effectively. See full prescribing information for Diovan. infarction, consideration should be given to a dosage reduction if symptomatic hypotension or renal dysfunction occurs. Diovan (valsartan) Tablets -----------DOSAGE FORMS AND STRENGTHS------------ Initial U.S. Approval: 1996 Tablets (mg): 40 (scored), 80, 160, 320 WARNING: USE IN PREGNANCY ---------------------CONTRAINDICATIONS------------------- When pregnancy is detected, discontinue Diovan as soon as possible. None Drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus (5.1) ---------------WARNINGS AND PRECAUTIONS------------ • Avoid fetal or neonatal exposure (5.1) -----------------RECENT MAJOR CHANGES----------------- • Observe for signs and symptoms of hypotension (5.2) Indications and Usage: Benefits of lowering blood pressure (1) 12/2011 • Use with caution in patients with impaired hepatic (5.3) or renal (5.4) function --------------INDICATIONS AND USAGE--------------------- Diovan is an angiotensin II receptor blocker (ARB) indicated for: -------------------ADVERSE REACTIONS ----------------- • Treatment of hypertension, to lower blood pressure. Lowering blood Hypertension: Most common adverse reactions are headache, dizziness, pressure reduces the risk of -

Angiotensin-Converting Enzyme (ACE) Inhibitors
Angiotensin-Converting Enzyme (ACE) Inhibitors Summary Blood pressure reduction is similar for the ACE inhibitors class, with no clinically meaningful differences between agents. Side effects are infrequent with ACE inhibitors, and are usually mild in severity; the most commonly occurring include cough and hypotension. Captopril and lisinopril do not require hepatic conversion to active metabolites and may be preferred in patients with severe hepatic impairment. Captopril differs from other oral ACE inhibitors in its rapid onset and shorter duration of action, which requires it to be given 2-3 times per day; enalaprilat, an injectable ACE inhibitor also has a rapid onset and shorter duration of action. Pharmacology Angiotensin Converting Enzyme Inhibitors (ACE inhibitors) block the conversion of angiotensin I to angiotensin II through competitive inhibition of the angiotensin converting enzyme. Angiotensin is formed via the renin-angiotensin-aldosterone system (RAAS), an enzymatic cascade that leads to the proteolytic cleavage of angiotensin I by ACEs to angiotensin II. RAAS impacts cardiovascular, renal and adrenal functions via the regulation of systemic blood pressure and electrolyte and fluid balance. Reduction in plasma levels of angiotensin II, a potent vasoconstrictor and negative feedback mediator for renin activity, by ACE inhibitors leads to increased plasma renin activity and decreased blood pressure, vasopressin secretion, sympathetic activation and cell growth. Decreases in plasma angiotensin II levels also results in a reduction in aldosterone secretion, with a subsequent decrease in sodium and water retention.[51035][51036][50907][51037][24005] ACE is found in both the plasma and tissue, but the concentration appears to be greater in tissue (primarily vascular endothelial cells, but also present in other organs including the heart). -

Comparative Effects of Telmisartan and Valsartan As Add-On Agents For
Hypertension Research (2014) 37, 225–231 & 2014 The Japanese Society of Hypertension All rights reserved 0916-9636/14 www.nature.com/hr ORIGINAL ARTICLE Comparative effects of telmisartan and valsartan as add-on agents for hypertensive patients with morning blood pressure insufficiently controlled by amlodipine monotherapy Hideaki Yoshida1, Hiroshi Akasaka1, Shigeyuki Saitoh1, Kazuaki Shimamoto1 and Tetsuji Miura1 on behalf of the SPEED investigators2 The aim of this study was to determine the efficacies of valsartan and telmisartan as add-on agents for the control of morning blood pressure (BP) in patients already on amlodipine monotherapy. A total of 414 hypertensive patients were prospectively enrolled in a 4-week run-in period when they were treated with amlodipine (5 mg/day), and home BP was measured in the morning and evening. Patients with home systolic BP (SBP) being 135–159 mm Hg in the morning at the end of the run-in period were randomized to additional treatment with valsartan (80 mg/day) or with telmisartan (40 mg/day) for 8 weeks. The primary endpoint was the change in morning home BP, and secondary endpoints included variability of morning home BP. Of the 282 patients randomized, 262 patients (n ¼ 131, in each treatment) completed the protocols. Demographic parameters and baseline morning SBP/diastolic BP (DBP) (146.3±7.1/84.8±9.3 vs. 146.0±7.1/84.2±9.1 mm Hg) were comparable in the valsartan group and telmisartan group, and changes in SBP/DBP after 8-week treatment were not significantly different between the two groups ( À7.4±10.6/ À3.9±6.1 vs. -

Is Enalapril and Losartan Combination Irrational?Irrational?
Correspondence Is enalapril and losartan combination irrational?irrational? I read with great interest the editorial by C.S. Gautam and of exogenous angiotensin I pressor effects.[4] Therefore, the S. Aditya, which appeared in the Indian Journal of enalapril-losartan combinations are more potent at Pharmacology in June 2006 (vol, 38(3):167-70). A very achieving these goals than any of their constituents important topic was addressed in the editorial, and I individually. compliment the authors on this. On the other hand, I felt � Similarly synergistic efficacy of enalapril and losartan in confused when I found Enalapril + Losartan in the list of combination on exercise performance and oxygen irrational fixed dose combinations. I agree with the authors consumption at peak exercise in congestive heart failure that combining the two drugs affecting the same pathway is has been sugested.[5] irrational as it does not add to its efficacy, but I am not sure � The combination of an angiotensin-converting enzyme whether this combination should be categorised as irrational inhibitor and an angiotensin II receptor antagonist (AT1 or as rational. The results of ongoing research may answer receptor antagonist) in patients can significantly enhance this question in future. reduction in left ventricular hypertrophy. This can provide Apparently this combination may appear irrational, but greater protection to the heart against the overload caused actually rationality does exist for combining these two drugs. by persistent hypertension. Furthermore, the combination � From a physiological point of view, the general mechanism of these drugs did not increase the incidence of adverse of action of both angiotensin-converting enzyme inhibitor effects. -

Shortage of Valsartan Formulations
Shortage of Valsartan Formulations Updated 24th September 2019 Description of products affected Valsartan is an angiotensin II receptor antagonist (AIIRA) licensed for the treatment of patients with: • Hypertension. • Symptomatic heart failure. • Post- myocardial infarction (MI) with left ventricular failure or left ventricular systolic dysfunction (LVSD). Licensed doses range from 40mg to 320mg per day given once or twice daily depending on indication. It is one of several AIIRAs on the UK market which are all licensed for the treatment of hypertension but differ in their other licensed indications. Background • There have been intermittent supply problems with all strengths of valsartan formulations (including both capsules and tablets). • Our Medicines Optimisation Team has managed to ascertain the following availability from manufacturers as of 20th September 2019: o Crescent – Valsartan 80mg capsules and valsartan 160mg capsules are available. o DE Pharmaceuticals – Valsartan 40mg tablets and valsartan 320mg tablets are available. o Mylan – Valsartan 160mg capsules and valsartan 320mg tablets are available. • There is currently only the broad anticipated resolution date of October to November 2019. • Suppliers of alternative AIIRAs have been contacted to determine if they have sufficient stock availability and currently both Losartan and Candesartan are available from the 3 major wholesalers: AAH, Alliance HC and Phoenix. Alternative agents and suggested management options • The patient should be encouraged to try several pharmacies in order to fulfil the prescription as different pharmacies use a range of wholesalers and distributors. The patient may wish to ring pharmacies in advance of attending to ascertain availability. Page 1 of 3 • The decision about what to do will need to be individualised to each patient. -

Switching Ace-Inhibitors
Switching Ace-inhibitors http://www.ksdl.kamsc.org.au/dtp/switching_ace_inhibitors.html Change to → Enalapril Quinapril Ramipril Change from ↓ (Once daily dosing) (Once daily dosing) (Once daily dosing) Captopril Captopril 12.5mg daily Enalapril 2.5mg1 Quinapril 2.5mg Ramipril 1.25mg Captopril 25mg daily Enalapril 5mg1 Quinapril 5mg Ramipril 1.25-2.5mg Captopril 50mg daily Enalapril 7.5mg1 Quinapril 10mg Ramipril 2.5-5mg Captopril 100mg daily Enalapril 20mg1 Quinapril 20mg Ramipril 5-10mg2 Captopril 150mg daily Enalapril 40mg Quinapril 40mg Ramipril 10mg Fosinopril Fosinopril 5mg daily Enalapril 5mg Quinapril 5mg Ramipril 1.25mg Fosinopril 10mg daily Enalapril 10mg Quinapril 10mg Ramipril 2.5mg Fosinopril 20mg daily Enalapril 20mg Quinapril 20mg Ramipril 5mg Fosinopril 40mg daily Enalapril 40mg Quinapril 40mg Ramipril 10mg Lisinopril Lisinopril 5mg daily Enalapril 5mg Quinapril 5mg Ramipril 1.25mg Lisinopril 10mg daily Enalapril 10mg Quinapril 10mg Ramipril 2.5mg Lisinopril 20mg daily Enalapril 20mg Quinapril 20mg Ramipril 5mg Lisinopril 40mg Enalapril 40mg Quinapril 40mg Ramipril 10mg Perindopril Perindopril 2mg daily Enalapril 5-10mg Quinapril 5-10mg Ramipril 2.5mg Perindopril 4mg daily Enalapril 10mg-20mg Quinapril 10mg-20mg Ramipril 5mg Perindopril 8mg daily Enalapril 20-40mg Quinapril 20-40mg Ramipril 10mg Trandolapril Trandolapril 0.5mg d Enalapril 5mg Quinapril 5mg Ramipril 1.25mg Trandolapril 1mg daily Enalapril 10mg Quinapril 10mg Ramipril 2.5mg Trandolapril 2mg daily Enalapril 20mg Quinapril 20mg Ramipril 5mg Trandolapril 4mg daily Enalapril 40mg Quinapril 40mg Ramipril 10mg There are few studies comparing equivalent doses of ACE-inhibitors, for specific indications. Therefore, the above recommendations are based on clinical experiences and are not specific for any indication. -

Download Leaflet View the Patient Leaflet in PDF Format
Package leaflet: Information for the user Valsartan 40 mg hard capsules Valsartan 80 mg hard capsules Valsartan 160 mg hard capsules valsartan Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. • Keep this leaflet. You may need to read it again. • If you have any further questions, ask your doctor or pharmacist. • This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. • If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Valsartan is and what it is used for 2. What you need to know before you take Valsartan 3. How to take Valsartan 4. Possible side effects 5. How to store Valsartan 6. Contents of the pack and other information 1. What Valsartan is and what it is used for Valsartan belongs to a class of medicines known as angiotensin II receptor antagonist, which help to control high blood pressure. Angiotensin II is a substance in the body that causes vessels to tighten, thus causing your blood pressure to increase. Valsartan works by blocking the effect of angiotensin II. As a result, blood vessels relax and blood pressure is lowered. Valsartan 40 mg hard capsules can be used for three different conditions: • to treat high blood pressure in children and adolescents 6 to 18 years of age. -

Valsartan: Pediatric Medication
PATIENT & CAREGIVER EDUCATION Valsartan This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US Diovan Brand Names: Canada ACT Valsartan [DSC]; APO-Valsartan; Auro-Valsartan; Diovan; DOM-Valsartan [DSC]; MYLAN-Valsartan [DSC]; PMS-Valsartan [DSC]; RIVA-Valsartan [DSC]; SANDOZ Valsartan; TARO-Valsartan; TEVA-Valsartan Warning If your child is or may be pregnant: Do not give this drug to your child during pregnancy. Use during pregnancy may cause birth defects or loss of the unborn baby. If your child gets pregnant or plans on getting pregnant while taking this drug, call the doctor right away. What is this drug used for? It is used to treat high blood pressure. It may be given to your child for other reasons. Talk with the doctor. Valsartan 1/7 What do I need to tell the doctor BEFORE my child takes this drug? If your child is allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell the doctor about the allergy and what signs your child had. If your child is taking a drug that has aliskiren in it and your child also has diabetes or kidney problems. If your child is breast-feeding a baby: Be sure your child does not breast-feed a baby while taking this drug. This is not a list of all drugs or health problems that interact with this drug. Tell the doctor and pharmacist about all of your child’s drugs (prescription or OTC, natural products, vitamins) and health problems. -

Valoración De La Terapia Combinada De Enalapril Con Irbesartán Sobre La Función Renal En Pacientes Diabéticos Tipo 2
Revista Médica de la Universidad de Costa Rica. Volumen 1, número 1, artículo 7, septiembre 2007. http://www.revistamedica.ucr.ac. Sección Estudiantil Valoración de la terapia combinada de Enalapril con Irbesartán sobre la función renal en pacientes diabéticos tipo 2 Rodríguez Hernández, Andrea.*, Rosenkrantz Amón, Shunit.*, Jiménez Loría, Federico**, Chaverri Fernández, José Miguel*** *Estudiantes de Farmacia. Universidad de Costa Rica, **Farmacéutico. Subdirector de Farmacia Hospital México, ***Farmacéutico. Centro Nacional de Información de Medicamentos. INIFAR. Facultad de Farmacia. Universidad de Costa Rica. Resumen: El presente trabajo pretende adicionar evidencia que respalde el uso concomitante de enalapril e irbersartán en los pacientes diabéticos del Hospital México. Métodos: Se seleccionó una muestra de pacientes diabéticos atendidos en el servicio de nefrología del Hospital México durante el periodo 2004-2006, valorándose en ellos, la función renal y la correlación existente con respecto al tratamiento recibido como nefroprotección, ya sea IECA o ARA II asociado a IECA. Resultados: La proporción de pacientes que utilizaron el tratamiento combinado y logran mejorar su función renal es mucho mayor que la proporción de pacientes que alcanzan esta mejoría dentro del grupo control o con monoterapia. Conclusiones: Los datos indican que existe una posible ventaja al utilizar el tratamiento combinado IECA – ARA II vs. la monoterapia con IECA como agentes renoprotectores. Palabras claves: Neuropatía diabética, ARA II, IECAS, diabetes, renoprotección. Recibido: Junio 2007. Aceptado: Septiembre. Publicado: Septiembre 2007. _____________________________________________________________________ 64 Revista electrónica publicada por la Escuela de Medicina de la Universidad de Costa Rica, 2060 San José, Costa Rica. ® All rights reserved. Revista Médica de la Universidad de Costa Rica.