Gmps for Apis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
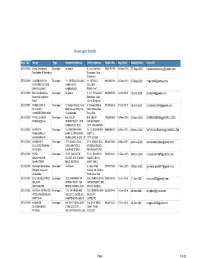
Developer Details
Developer Details Reg. No. Name Type Resident Address Office Address Mobile No. Reg. Date Validity Date Email ID DEV/00898 Zarna Developers Developer as above 5, Shiv Darshan 9825190196 28-Sep-2015 27-Sep-2020 [email protected] Sandipbhai B Kakadiya Bunglows, Opp. Shreeram DEV/00899 LAXMINARAYAN Developer 11, GIRIRAJ COLONY, 11, GIRIRAJ 9825034144 28-Sep-2015 27-Sep-2020 [email protected] INFRASRTUCUTRE PANCHVATI, COLONY, MANOJKUMAR AAMBAWADI, PANCHVATI, DEV/00900 Shrinand Buildcon Developer as above 1, S.F, Shreedhar 9825061073 16-Oct-2015 15-Oct-2020 [email protected] Jayantilal Nagjibhai Bunglows, Opp. Patel Grand Bhagwati, DEV/00901 SHREE SARJU Developer 9, Swagat Mahal, Near 9, Swagat Mahal, 9727442416 27-Oct-2015 26-Oct-2020 [email protected] BUILDERS Matrushree Party Plot, Near Matrushree CHANDRESHKUMAR Chandkheda , Party Plot, DEV/00902 PATEL DHIREN Developer B-6, MILAP B-6, MILAP 7096638633 03-Nov-2015 02-Nov-2020 [email protected] PRAHLADBHAI APPARTMENT, OPP. APPARTMENT, RANAKPUR SOC, OPP. RANAKPUR DEV/00903 SATASIYA Developer 14, SUROHI PARK 14, SUROHI PARK 9898088520 05-Nov-2015 04-Nov-2020 [email protected] PRAKASHBHAI PART-2,OPP.SUKAN PART-2, KARSHANBHAI BUNGLOWS AUDA T.P OPP.SUKAN DEV/00904 KARNAVATI Developer 17/A, KAMLA SOC, 17/A, KAMLA SOC, 9824015660 24-Nov-2015 23-Nov-2020 [email protected] BUILDERS RAMANI STADIUM ROAD, STADIUM ROAD, BHISHAM J NAVRANGPURA, NAVRANGPURA, DEV/00905 PATEL Developer F/101, SANGATH F/101, SANGATH 9925018327 01-Dec-2015 30-Nov-2020 [email protected] MALAYKUMAR SILVER, B/H D MART SILVER, B/H D BHARATBHAI MALL MOTERA, MART MALL DEV/00906 Harikrupa Developers Developer As Above 6, Ishan Park 7874377897 11-Dec-2015 10-Dec-2020 [email protected] Prajapati Jaymesh Society, Nr. -

Behrouz Biryani
Online Offer – Behrouz Biryani: • G 42, Shree Mahalaxmi Shops, Rudra Square, Bodakdev, Ahmedabad • 25, Rivera Arcade, Near Prahlad Nagar Garden, Prahlad Nagar, Ahmedabad • 2, IM Complex, Vastrapur Lake, Vastrapur, Ahmedabad • G/F 1, Animesh Complex, Panchavati Ellis Bridge, Near Chandra Colony, C G Road, Ahmedabad • 14, Ground Floor, Vitthal the Mall, Near Swagat Status, Chandkheda • C-19-20 Swagat Rainforest 2, Village Kudasan, Ta and District, Airport Gandhinagar Highway, Gandhinagar, Ahmedabad • 7th Cross Road, 8th Main, BTM Layout, Bangalore • Near Sony World Signal, Koramangala 6th Block, Bangalore • Kodichikkanahalli Main Road, Begur Hobli, Bommanahalli, Bangalore • Ground Floor, Actove Hotel, Kadubisanahalli, Marathahalli, Bangalore • Food Court, Sjr I-Park, Built In, Whitefield, Bangalore • Old Airport Road, Old Airport Road, Bangalore • Devatha Plaza, Residency Road, Bangalore • 123, Kamala Complex, AECS Layout, ITPL Main Road, Whitefield, Bangalore • 2318, Sector 1, Near NIFT College, HSR Layout, Bangalore • Kaggadaspura, CV Raman Nagar, Bangalore • Shop A-94 6/2, Opposite State Bank of India, 2nd Phase, J P Nagar, Bangalore • 101, Ground Floor, Manjunatha Complec, 22nd Main Road, 2nd Stage, Banashankari, Bangalore • Site No 8, New No 1, Channasandra, Property No 121, 2nd Main Road, Kr Puram Hobli, Kasturi Nagar, Bangalore • Shop 10-11, Electronic City Phase-1, 2nd Cross Road, Near Infosys Gate 1, Bangalore • 2283, 1st Main Road, Sahakar Nagar D Block, Bangalore • 3, 1st Floor, Apple City, Kadugodi Hoskote, Main Road, Seegehalli, Bangalore • Shop No. 837, BEML 3rd Stage, Halagevaderahalli, Rajarajeshwari Nagar, Bangalore • Dodaballapur Main Road, Puttenahalli, Yelahanka, Bangalore • Colony Skylineapartment, Canara Bank, Chandra Layout, Bangalore • Shop No. 90, First Floor, Sanjay Nagar Main Road, Geddalahalli, Bangalore • 6, First Floor, 9 Cross, 2nd Main, Binnamangala, 1st Stage, Indiranagar, Bangalore • Shop No. -

Marda Collections
Brand City Address (Marda Collections) UGF , M.L Plaza , Post Office, Chowmuhani, Mantribari Rd Ext, GLOBAL DESI Agartala Dhaleshwar, Agartala, Tripura 799001 And Designs - Gulmohar Park, G - 10, Ground Floor, Near Fun Republic, Satellite Road, AND Ahmedabad Ahmedabad - 380 00 K L Fashions - (And), Unit No. F - 23, First Floor, Alpha One Mall, Tpn. 1, Fp No. 261, AND Ahmedabad Vastrapur Lake, Ahmedabad Shop No. G11, Ground Floor, Gulmohar Park Mall, Near Fun re Public, Satellite Road, GLOBAL DESI Ahmedabad Ahmedabad - 380 GLOBAL DESI Ahmedabad 28, First Floor, Alpha One, Vastrapur Lake. Ahmedabad - 380054 AND+GLOBAL DESI Ahmedabad Shoppers Plaza, Nr. Peter England, CG Road, Ahmedabad - 380009 Shop No. 3, Ground Floor, Venus Atlantis, 100 Ft, Prahladnagar Road, Satellite, AND+GLOBAL DESI Ahmedabad Ahmedabad – 380015, Gujarat. GLOBAL DESI Aligarh Shop No 1, Ayodhya Kutti, Opposite Abdullah College,Marris Road, Aligarh - 202001 Abacus Retail, Shop No 81/32, Lal Bahadur Shastri Nagar, Civil Lines, Allahabad, Uttar AND+GLOBAL DESI Allahabad Pradesh - 211001 Unit No.21, UGF, B Wing, Trilium Mall, Circular Road, Basant Avenue, Amritsar, Punjab - AND Amritsar 143001 Ground Floor, Raghuvir City Centre, Bhalej Rd, Gamdi Vad, Sardar Ganj Anand, Gujarat AND+GLOBAL DESI Anand 388001 Ground 2: G2 - 15, Inorbit Mall, Whitefield, No. 75, EPIP Area, Whitefield, Bengaluru AND Bangalore 560066. Shop No. 209, Second Floor, Garuda Mall, Commissariat Road, Magrath Road Junction, AND Bangalore Bengaluru - 560025. AND Bangalore The High Street, 11th Main Road, 4th Block, Jayanagar, Bangalore - 560 011 Plot No. 11B, Survey No. 40/9, Dyvasandra Village, Krishna Raj Puram, Hobli, Bangalore AND Bangalore East Taluk, Bangalore - 560 048 Unit No. -

Ecological Investigations of Shahwadi Wetland Nainesh R
Explorer Research Article [Modi et al. , 4(12): Dec., 2013] CODEN (USA): IJPLCP ISSN: 0976-7126 INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES Ecological investigations of Shahwadi Wetland Nainesh R. Modi 1*, Nilesh R. Mulia 1 and Sumesh N. Dudani 2 1, Department of Botany, M. G. Science Institute, Ahmedabad, (Gujarat) - India 2, Deparment of Botany, Yuvaraja’s College, University of Mysore, Mysore - India Abstract The wetlands form important repositories of aquatic biodiversity. The diverse ecoclimatic regimes extant in the country resulted in a variety of wetland systems ranging from high altitude cold desert wetlands to hot and humid wetlands in coastal zones with its diverse flora and fauna. There are many important lakes and wetlands in Ahmedabad including Kankaria lake and Vastrapur Lake. The Nalsarovar wetland and Thol lake in the outskirts of Ahmedabad city are very important spots for migratory birds coming from different parts of the world and hence, attracts large number of tourists. However, many small and big wetlands are also present in and around the city which has not received due attention and hence, have suffered badly in the increased age of industrialization and urbanization. One such wetland is Shahwadi wetland which is situated in the heavily industrialized Narol area of Ahmedabad city. This study was undertaken to understand the ecological richness and importance of this region and highlight its degrading condition. It was found that this wetland harbors a good number of migratory birds and local birds, but is in highly degrading condition due to the negligence of local people residing in the periphery and the industries running nearby. -

The Spectre of SARS-Cov-2 in the Ambient Urban Natural Water in Ahmedabad and Guwahati: a Tale of Two Cities
medRxiv preprint doi: https://doi.org/10.1101/2021.06.12.21258829; this version posted June 16, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . The Spectre of SARS-CoV-2 in the Ambient Urban Natural Water in Ahmedabad and Guwahati: A Tale of Two Cities Manish Kumar1,2*, Payal Mazumder3, Jyoti Prakash Deka4, Vaibhav Srivastava1, Chandan Mahanta5, Ritusmita Goswami6, Shilangi Gupta7, Madhvi Joshi7, AL. Ramanathan8 1Discipline of Earth Science, Indian Institute of Technology Gandhinagar, Gujarat 382 355, India 2Kiran C Patel Centre for Sustainable Development, Indian Institute of Technology Gandhinagar, Gujarat, India 3Centre for the Environment, Indian Institute of Technology Guwahati, Assam 781039, India 4Discipline of Environmental Sciences, Gauhati Commerce College, Guwahati, Assam 781021, India 5Department of Civil Engineering, Indian Institute of Technology Guwahati, Assam 781039, India 6Tata Institute of Social Science, Guwahati, Assam 781012, India 7Gujarat Biotechnology Research Centre (GBRC), Sector- 11, Gandhinagar, Gujarat 382 011, India 8School of Environmental Sciences, Jawaharlal Nehru University, New Delhi 110067, India *Corresponding Author: [email protected]; [email protected] Manish Kumar | Ph.D, FRSC, JSPS, WARI+91 863-814-7602 | Discipline of Earth Science | IIT Gandhinagar | India 1 NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2021.06.12.21258829; this version posted June 16, 2021. -

Thaltej Village: an Incremental Approach to Urban Encroachment
Thaltej Village: An Incremental Approach to Urban Encroachment Thaltej Village: An Incremental Approach to Urban Encroachment Emily Brown Allison Buchwach Ryan Hagerty Mary Richardson Laura Schultz Bin Yan Under the advisement of Professor Michael Dobbins Georgia Institute of Technology April 27, 2012 Acknowlegements This report was produced with help from faculty and students at CEPT University in Ahmdebad, as well as many other generous folks both here and abroad that have helped us immeasurably with their advice, insight and feedback along the way. To all, we extend our heartfelt gratitude. Contents 1 INTRODUCTION ............................................................................................................................................. 1 2 INDIAN NATIONAL CONTEXT ......................................................................................................................... 3 2.1 INDIA’S URBANIZATION AND ITS IMPACT ON SLUMS AND THE ENVIRONMENT ................................................................ 3 2.2 IMPACT OF URBANIZATION: ENVIRONMENTAL DEGRADATION .................................................................................... 5 2.3 POLICY RESPONSES ............................................................................................................................................ 6 2.4 POLICY RESPONSES ............................................................................................................................................ 8 2.4.1 Slum Clearance (1956) ............................................................................................................................ -

AMDHY Fact Sheet
NEAR VASTRAPUR LAKE, VASTRAPUR Ahmedabad, Gujarat 380015 India T + (9179) 6160 1234 F + (9179) 6160 1235 [email protected] ahmedabad.hyatthotels.hyatt.com you’re more than welcome 2013.02 Accommodations Services & Facilities • 178 rooms including 10 suites • 24-hour in-room dining • Size of the rooms range from 26 to 111.48 square metres • Airport limousine / limousine for hire (280 to 1,200 square feet) • Car rental • Choice of smoking and non-smoking rooms • 24-hour Assistant Manager / Concierge • Ergonomically designed beds • Car parking with valet parking facilities • Range of comfort pillows • Doctor on call • Bedside electronic controls • Currency exchange • Spacious bathroom with a walk-in shower cubicle • Executive Lounge for Executive room and Suite room guests (bath tub facility in Hyatt executive Suite only) • Bathroom amenities and hairdryer Conferences & Banquets • Tea- and coffee- making facilities • State-of-the-art conference and banquet facilities ideal for • Stocked minibar (optional health minibar) board meetings, closed door discussion, break away functions, • Individually controlled air-conditioning and lighting weddings and banquets are available at lobby level with • Large bay windows 1,068.39 square metres (11,500 square feet) of space • 32-inch LCD television with international channels • A pre-function area and two separate meeting rooms (40-inch LCD television in Suite Rooms) • Broadband data port / wired and wireless Internet accommodating smaller meetings • Iron / ironing board on request • Meeting areas are equipped with the latest software, Wi- • In-room electronic safes conferencing capabilities Recreational Facilities Restaurant, Bar & Lounge • Spa — featuring seven beautifully appointed rooms with • Collage — designed to be an uplifting yet informal experience, interconnecting shower cubicles, including a salon. -

Gujarat State
CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES, RIVER DEVELOPMENT AND GANGA REJUVENEATION GOVERNMENT OF INDIA GROUNDWATER YEAR BOOK – 2018 - 19 GUJARAT STATE REGIONAL OFFICE DATA CENTRE CENTRAL GROUND WATER BOARD WEST CENTRAL REGION AHMEDABAD May - 2020 CENTRAL GROUND WATER BOARD MINISTRY OF WATER RESOURCES, RIVER DEVELOPMENT AND GANGA REJUVENEATION GOVERNMENT OF INDIA GROUNDWATER YEAR BOOK – 2018 -19 GUJARAT STATE Compiled by Dr.K.M.Nayak Astt Hydrogeologist REGIONAL OFFICE DATA CENTRE CENTRAL GROUND WATER BOARD WEST CENTRAL REGION AHMEDABAD May - 2020 i FOREWORD Central Ground Water Board, West Central Region, has been issuing Ground Water Year Book annually for Gujarat state by compiling the hydrogeological, hydrochemical and groundwater level data collected from the Groundwater Monitoring Wells established by the Board in Gujarat State. Monitoring of groundwater level and chemical quality furnish valuable information on the ground water regime characteristics of the different hydrogeological units moreover, analysis of these valuable data collected from existing observation wells during May, August, November and January in each ground water year (June to May) indicate the pattern of ground water movement, changes in recharge-discharge relationship, behavior of water level and qualitative & quantitative changes of ground water regime in time and space. It also helps in identifying and delineating areas prone to decline of water table and piezometric surface due to large scale withdrawal of ground water for industrial, agricultural and urban water supply requirement. Further water logging prone areas can also be identified with historical water level data analysis. This year book contains the data and analysis of ground water regime monitoring for the year 2018-19. -

Through Development Plan & Town Planning Scheme
Land Pooling and Land Management Through Development Plan & Town Planning Scheme Ahmedabad Urban Development Authority September 2014 Contents of the Presentation Introduction First Tier Planning Process - Development Plan Second Tier Planning Process - Town Planning Scheme (Self Financing Mechanism) Town Planning Scheme Procedure - Physical Planning Town Planning Scheme Procedure - Fiscal Planning Town Planning Scheme : An Efficient and Effective Tool To Implement Development Plan Land Management Findings Introduction Urbanization in Gujarat • Third Most urbanized State with 37.35 % of Urban Population as against 27.78% of India. • 167 Urban Local Bodies • Ahmedabad is 7th largest Urban Agglomeration in India. • Third Fastest Growing Cities in the World Ahmedabad in a Regional Context • Sanand SIR • Changodar SIR. • Gandhinagar. • 5 Growth Centers. History of Town Planning • The Bombay Town Planning Act 1915: Provision of Town Planning Scheme • Bombay Town Planning Act 1954 : The Provision of Development Plan added. • Gujarat Town Planning & Urban Development Act,1976 Provision of Planning the Urban Development Area/ Authority. TPS 2 Kankaria Sanctioned DP 1965 Sanctioned DP 2011 (AUDA) Challenges to Urban Planning • Implementation of Development Plan / Master Plan • Implementation of Regional, City and Neighborhood Level Physical and Social Infrastructure • Land Bank for Urban Poor • Resource Generation and Mobilization in terms of Physical / Land • Resource Generation and Mobilization in terms of Fiscal / Finance • Improving and Maintaining -

Shri Maharaja Agrasen Seva Sansthan Members List
SHRI MAHARAJA AGRASEN SEVA SANSTHAN MEMBERS LIST FORM NAME OF MEMBER R C0R0SPONDENCE ADDRESS GOTRA CONTACT NUMBERS NO E S I 1 1.SHRI SOMNATH R GUPTA B-B-101, Ratnakar-3, Near KalaDarsan, Prernatirth, GOYAL O26922484, M9825346308, 1Anandnagar Road, Ahmedabad - 15 M9825024240 0 1 2 2.SHRI SUNIL M AGRAWAL 112/A-GREENFIELD,OPP-MURLI MANGAL M-9824048050 2RESTAURANT,SINDHUBHAVAN /ROAD,THALTEJ,AHMEDABAD-380059 A 3 3.SHRI KAILASH B AGRAWAL B-B-59, KONARK TOWER, B/H., NALANDA SHOPPING , MANGAL R&O.26765583 M.9898094889 5SATTELITE, AHMEDABAD.-15 9 , 4 4.SHRI RAJEEV C AGRAWAL B-B-2, SHIV TOWER, RAMDEVNAGAR, SATELLITE, GOYAL R40065346, O26925346, 2AHMEDABAD-15 M9825007199 , S 5 5.SHRI GIRDHARILAL S GUPTA 770/71, GREENPARK BUNGLOWS, AMBLI VILLAGE, BOPAL GOYAL R02717-235605, M9427702449 0RD., AHMEDABAD- / 7 6 6.SHRI SHARAD R AGRAWAL B-B-2 NAMAN APPARTMENT, OPP SAMBHAV PRESS, GARG O26870357, R26870586, 2BODAKDEV, AHMEDABAD 15 M9825933890, M9427704817 N A 7 7.SHRI RAMAVTAR P B-B-201,AROHI AGHA , NR GRAND BHAGWATI SINGHAL O22169164, R27551382, CHAUDHARI 2HOTE,OPP.GURUDWARA,S.G ROAD, AHMEDABAD. R27552286, M9375055544 0 1 8 8.SHRI VIJAYKUMAR R GUPTA 220, RIVERA BUGLOWS, RIVERA20, NR PRAHLADNAGAR GARG O29296681, R29296661, 0AUDA GARDAN, AHMEDABAD - 51 M9825010706 , R 9 9.SHRI DWARKAPRASAD G OOFFICE: A/401, SAMUDRA COMPLEX, NEAR CLASSIC O26440740, R26855696, BAJAJ FGOLD, ELLISBRIDGE, AHMEDABAD 6 M9825005303 F I 10 10.SHRI POPATLAL B 117, DEVPRIYA BUNGLOWS, PART-II, NR AGRAWAL O22170330, R29097600, AGRAWAL 7TOWER, ANANDNAGAR CROSS ROAD, AHMEDABAD M9825062852 , D 11 11.SHRI KAILASHCHANDRA G B- M9898029192 GUPTA 6 0 1 9/27/2016 Page 1 SHRI MAHARAJA AGRASEN SEVA SANSTHAN MEMBERS LIST FORM NAME OF MEMBER R C0R0SPONDENCE ADDRESS GOTRA CONTACT NUMBERS NO E S 12 12.SHRI DHARMENDRA ISB-101 PEARL APPT , OPP SHAKTI SCHOOL SHYAMAL GOYAL O7600038393, M9979893830 GUPTA TCROSS ROAD, AHMEDABAD - 15 A T 13 13.SHRI RAVIPRAKASH GOYAL B-B-82, SHAKTI ENCLAVE, JUDGES BUNGLOW ROAD, GOYAL R26855975 M9374915156 8A'BAD-54 2 , 14 14.SHRI JITENDRA R B-B-10, PRAYAN APT., NR. -

Linking Urban Lakes
LINKING URBAN LAKES: Assessment of Water Quality and its Environmental Impacts AKSHAY ANAND February, 2014 SUPERVISORS: Ir. M.J.G. Mark Brussel Ms. M. Kuffer LINKING URBAN LAKES: Assessment of Water Quality and its Environmental Impacts AKSHAY ANAND Enschede, The Netherlands, [February, 2014] Thesis submitted to the Faculty of Geo-Information Science and Earth Observation of the University of Twente in partial fulfilment of the requirements for the degree of Master of Science in Geo-information Science and Earth Observation. Specialization: Urban Planning and Management SUPERVISORS: Ir. M.J.G. Mark Brussel Ms. M. Kuffer THESIS ASSESSMENT BOARD: Ms. Prof. dr. ing. P.Y. Georgiadou Dr. ir. C.M.M. Mannaerts [ University of Twente ] DISCLAIMER This document describes work undertaken as part of a programme of study at the Faculty of Geo-Information Science and Earth Observation of the University of Twente. All views and opinions expressed therein remain the sole responsibility of the author, and do not necessarily represent those of the Faculty. ABSTRACT Lakes in urban and peri-urban areas are an important interface between planning and ecology, which demands environmentally responsive strategies, acknowledging problems like flooding, water pollution, and water quality with their complexities in design and engineering. The present study attempts to investigate the impacts of hydrological planning interventions on lake ecosystems. The research highlights the issues in experimental projects like ‘lake linking project’ carried out by Ahmedabad Urban Development Authority (AUDA). The integration of storm water infrastructure and lake ecosystem creates adverse pressure on lake water quality which is subsequently also transferred to other connected lakes. -

THE BHAGYODAYA CO-OPERATIVE BANK LTD. Unclaimed Deposit Accounts Transfer to RBI DEA Fund Scheme 2014 As on 31-Jan-2020 SR NO
THE BHAGYODAYA CO-OPERATIVE BANK LTD. Unclaimed Deposit Accounts transfer to RBI DEA Fund Scheme 2014 as on 31-Jan-2020 SR NO. NAME OF ACCOUNT HOLDER ADDRESS 1. KITCHEN NEEDS DEVINA SHERI NA NAKE MANDVI NI POLE MANEKCHOWK 0 AHMEDABAD 2 3-D FOOD JUNCTION DEVDARSHAN,JAY HIND CHAR RASTA MANINAGAR,AHMEDABAD-380 008 0 3A & A SECURITIES B-16,SAMARPAN TOWER- NR SIMANDHAR COMP,K.K.NAGAR RD 380061 4 A ONE MASALA MILL 0 AHMEDABAD 5 A P PATEL[MINOR] 0 AHMEDABAD 6 A TO Z GROUP TUTIONS 7,ANAND MANGAL FLATES NR.KENYUG-5,SATELLITE 0 AHMEDABAD 7 A.A. PATEL FAMILY TRUST 0 AHMEDABAD 8 A.N.BIKE'S AND MOTORS LAXMI NAGAR SOCIETY, NAVA VADEJ, 0 AHMEDABAD 9 AADHAR CONSTRUCTION 337,K.K.NAGAR-4,RANNA PARK, GHATLODIA, 0 AHMEDABAD 10 AADIL CORPORATION 121/42,OPP-VICTORIA ARYAN WORK CHHOTALAL NI CHALI, 380004 11AADIL TRADERS OPP.VICTORIA IRON WORKS, CHHOTALAL NI CHALI,LOKHAND 380004 12 AAI SHREE KHODIYAR PETROLEUM AT - TRIKAMPURA, POST- VISALPUR, TAL.- DASCROI, 382210 AHMEDABAD 13 AAKRUTI OFFSET 41,MAHESHWARI ESTATE SHAHIBAUG 0 AHMEDABAD 14 AALEKH CONSULTANCY 7,AJITNATH SOCIETY FATEHNAGAR PALDI 380007 AHMEDABAD 15 AALEKH FINANCE 7.AJITNATH SOC PALDI 0 AHMEDABAD 16 AALEKH INVESTMENT C/O AANGI APP. N.V.G. ROAD PALDI 0 AHMEDABAD 17 AALEKH ORGANISER 7,AJITNATH SOCI.FATEHNAGAR. PALDI. 0 AHMEDABAD 18 AARAMBH JEWELLERS 5,AARAMBH COMPLEX, ANKUR CROSS ROAD 380013 AHMEDABAD 19 AARTI KHANIJO 18,SAKAN TWINS, MANAGEMENT ENCLOVE 380015 AHMEDABAD 20 AARTI MAULINBHAI TRIVEDI AT - RANDHEJA, GANDHINAGAR, 382010 AHMEDABAD 21 AARTI UMESH RAVAL C/5,MAHAVIRNAGAR FLATS,OPP.