Introduction to Image Analysis with FIJI
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
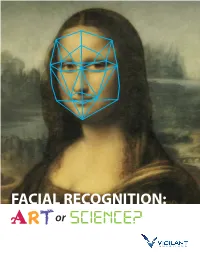
FACIAL RECOGNITION: a Or FACIALRT RECOGNITION: a Or RT Facial Recognition – Art Or Science?
FACIAL RECOGNITION: A or FACIALRT RECOGNITION: A or RT Facial Recognition – Art or Science? Both the public and law enforcement often misunderstand facial recognition technology. Hollywood would have you believe that with facial recognition technology, law enforcement can identify an individual from an image, while also accessing all of their personal information. The “Big Brother” narrative makes good television, but it vastly misrepresents how law enforcement agencies actually use facial recognition. Additionally, crime dramas like “CSI,” and its endless spinoffs, spin the narrative that law enforcement easily uses facial recognition Roger Rodriguez joined to generate matches. In reality, the facial recognition process Vigilant Solutions after requires a great deal of manual, human analysis and an image of serving over 20 years a certain quality to make a possible match. To date, the quality with the NYPD, where he threshold for images has been hard to reach and has often spearheaded the NYPD’s first frustrated law enforcement looking to generate effective leads. dedicated facial recognition unit. The unit has conducted Think of facial recognition as the 21st-century evolution of the more than 8,500 facial sketch artist. It holds the promise to be much more accurate, recognition investigations, but in the end it is still just the source of a lead that needs to be with over 3,000 possible matches and approximately verified and followed up by intelligent police work. 2,000 arrests. Roger’s enhancement techniques are Today, innovative facial now recognized worldwide recognition technology and have changed law techniques make it possible to enforcement’s approach generate investigative leads to the utilization of facial regardless of image quality. -

Statement by the Association of Hoving Image Archivists (AXIA) To
Statement by the Association of Hoving Image Archivists (AXIA) to the National FTeservation Board, in reference to the Uational Pilm Preservation Act of 1992, submitted by Dr. Jan-Christopher Eorak , h-esident The Association of Moving Image Archivists was founded in November 1991 in New York by representatives of over eighty American and Canadian film and television archives. Previously grouped loosely together in an ad hoc organization, Pilm Archives Advisory Committee/Television Archives Advisory Committee (FAAC/TAAC), it was felt that the field had matured sufficiently to create a national organization to pursue the interests of its constituents. According to the recently drafted by-laws of the Association, AHIA is a non-profit corporation, chartered under the laws of California, to provide a means for cooperation among individuals concerned with the collection, preservation, exhibition and use of moving image materials, whether chemical or electronic. The objectives of the Association are: a.) To provide a regular means of exchanging information, ideas, and assistance in moving image preservation. b.) To take responsible positions on archival matters affecting moving images. c.) To encourage public awareness of and interest in the preservation, and use of film and video as an important educational, historical, and cultural resource. d.) To promote moving image archival activities, especially preservation, through such means as meetings, workshops, publications, and direct assistance. e. To promote professional standards and practices for moving image archival materials. f. To stimulate and facilitate research on archival matters affecting moving images. Given these objectives, the Association applauds the efforts of the National Film Preservation Board, Library of Congress, to hold public hearings on the current state of film prese~ationin 2 the United States, as a necessary step in implementing the National Film Preservation Act of 1992. -

Lab 11: the Compound Microscope
OPTI 202L - Geometrical and Instrumental Optics Lab 9-1 LAB 9: THE COMPOUND MICROSCOPE The microscope is a widely used optical instrument. In its simplest form, it consists of two lenses Fig. 9.1. An objective forms a real inverted image of an object, which is a finite distance in front of the lens. This image in turn becomes the object for the ocular, or eyepiece. The eyepiece forms the final image which is virtual, and magnified. The overall magnification is the product of the individual magnifications of the objective and the eyepiece. Figure 9.1. Images in a compound microscope. To illustrate the concept, use a 38 mm focal length lens (KPX079) as the objective, and a 50 mm focal length lens (KBX052) as the eyepiece. Set them up on the optical rail and adjust them until you see an inverted and magnified image of an illuminated object. Note the intermediate real image by inserting a piece of paper between the lenses. Q1 ● Can you demonstrate the final image by holding a piece of paper behind the eyepiece? Why or why not? The eyepiece functions as a magnifying glass, or simple magnifier. In effect, your eye looks into the eyepiece, and in turn the eyepiece looks into the optical system--be it a compound microscope, a spotting scope, telescope, or binocular. In all cases, the eyepiece doesn't view an actual object, but rather some intermediate image formed by the "front" part of the optical system. With telescopes, this intermediate image may be real or virtual. With the compound microscope, this intermediate image is real, formed by the objective lens. -

Image Compression Using Discrete Cosine Transform Method
Qusay Kanaan Kadhim, International Journal of Computer Science and Mobile Computing, Vol.5 Issue.9, September- 2016, pg. 186-192 Available Online at www.ijcsmc.com International Journal of Computer Science and Mobile Computing A Monthly Journal of Computer Science and Information Technology ISSN 2320–088X IMPACT FACTOR: 5.258 IJCSMC, Vol. 5, Issue. 9, September 2016, pg.186 – 192 Image Compression Using Discrete Cosine Transform Method Qusay Kanaan Kadhim Al-Yarmook University College / Computer Science Department, Iraq [email protected] ABSTRACT: The processing of digital images took a wide importance in the knowledge field in the last decades ago due to the rapid development in the communication techniques and the need to find and develop methods assist in enhancing and exploiting the image information. The field of digital images compression becomes an important field of digital images processing fields due to the need to exploit the available storage space as much as possible and reduce the time required to transmit the image. Baseline JPEG Standard technique is used in compression of images with 8-bit color depth. Basically, this scheme consists of seven operations which are the sampling, the partitioning, the transform, the quantization, the entropy coding and Huffman coding. First, the sampling process is used to reduce the size of the image and the number bits required to represent it. Next, the partitioning process is applied to the image to get (8×8) image block. Then, the discrete cosine transform is used to transform the image block data from spatial domain to frequency domain to make the data easy to process. -

Ground-Based Photographic Monitoring
United States Department of Agriculture Ground-Based Forest Service Pacific Northwest Research Station Photographic General Technical Report PNW-GTR-503 Monitoring May 2001 Frederick C. Hall Author Frederick C. Hall is senior plant ecologist, U.S. Department of Agriculture, Forest Service, Pacific Northwest Region, Natural Resources, P.O. Box 3623, Portland, Oregon 97208-3623. Paper prepared in cooperation with the Pacific Northwest Region. Abstract Hall, Frederick C. 2001 Ground-based photographic monitoring. Gen. Tech. Rep. PNW-GTR-503. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 340 p. Land management professionals (foresters, wildlife biologists, range managers, and land managers such as ranchers and forest land owners) often have need to evaluate their management activities. Photographic monitoring is a fast, simple, and effective way to determine if changes made to an area have been successful. Ground-based photo monitoring means using photographs taken at a specific site to monitor conditions or change. It may be divided into two systems: (1) comparison photos, whereby a photograph is used to compare a known condition with field conditions to estimate some parameter of the field condition; and (2) repeat photo- graphs, whereby several pictures are taken of the same tract of ground over time to detect change. Comparison systems deal with fuel loading, herbage utilization, and public reaction to scenery. Repeat photography is discussed in relation to land- scape, remote, and site-specific systems. Critical attributes of repeat photography are (1) maps to find the sampling location and of the photo monitoring layout; (2) documentation of the monitoring system to include purpose, camera and film, w e a t h e r, season, sampling technique, and equipment; and (3) precise replication of photographs. -

An Introduction to Image Analysis Using Imagej
An introduction to image analysis using ImageJ Mark Willett, Imaging and Microscopy Centre, Biological Sciences, University of Southampton. Pete Johnson, Biophotonics lab, Institute for Life Sciences University of Southampton. 1 “Raw Images, regardless of their aesthetics, are generally qualitative and therefore may have limited scientific use”. “We may need to apply quantitative methods to extrapolate meaningful information from images”. 2 Examples of statistics that can be extracted from image sets . Intensities (FRET, channel intensity ratios, target expression levels, phosphorylation etc). Object counts e.g. Number of cells or intracellular foci in an image. Branch counts and orientations in branching structures. Polarisations and directionality . Colocalisation of markers between channels that may be suggestive of structure or multiple target interactions. Object Clustering . Object Tracking in live imaging data. 3 Regardless of the image analysis software package or code that you use….. • ImageJ, Fiji, Matlab, Volocity and IMARIS apps. • Java and Python coding languages. ….image analysis comprises of a workflow of predefined functions which can be native, user programmed, downloaded as plugins or even used between apps. This is much like a flow diagram or computer code. 4 Here’s one example of an image analysis workflow: Apply ROI Choose Make Acquisition Processing to original measurement measurements image type(s) Thresholding Save to ROI manager Make binary mask Make ROI from binary using “Create selection” Calculate x̄, Repeat n Chart data SD, TTEST times and interpret and Δ 5 A few example Functions that can inserted into an image analysis workflow. You can mix and match them to achieve the analysis that you want. -

A Review on Image Segmentation Clustering Algorithms Devarshi Naik , Pinal Shah
Devarshi Naik et al, / (IJCSIT) International Journal of Computer Science and Information Technologies, Vol. 5 (3) , 2014, 3289 - 3293 A Review on Image Segmentation Clustering Algorithms Devarshi Naik , Pinal Shah Department of Information Technology, Charusat University CSPIT, Changa, di.Anand, GJ,India Abstract— Clustering attempts to discover the set of consequential groups where those within each group are more closely related to one another than the others assigned to different groups. Image segmentation is one of the most important precursors for image processing–based applications and has a crucial impact on the overall performance of the developed systems. Robust segmentation has been the subject of research for many years, but till now published work indicates that most of the developed image segmentation algorithms have been designed in conjunction with particular applications. The aim of the segmentation process consists of dividing the input image into several disjoint regions with similar characteristics such as colour and texture. Keywords— Clustering, K-means, Fuzzy C-means, Expectation Maximization, Self organizing map, Hierarchical, Graph Theoretic approach. Fig. 1 Similar data points grouped together into clusters I. INTRODUCTION II. SEGMENTATION ALGORITHMS Images are considered as one of the most important Image segmentation is the first step in image analysis medium of conveying information. The main idea of the and pattern recognition. It is a critical and essential image segmentation is to group pixels in homogeneous component of image analysis system, is one of the most regions and the usual approach to do this is by ‘common difficult tasks in image processing, and determines the feature. Features can be represented by the space of colour, quality of the final result of analysis. -

The Microscope Parts And
The Microscope Parts and Use Name:_______________________ Period:______ Historians credit the invention of the compound microscope to the Dutch spectacle maker, Zacharias Janssen, around the year 1590. The compound microscope uses lenses and light to enlarge the image and is also called an optical or light microscope (vs./ an electron microscope). The simplest optical microscope is the magnifying glass and is good to about ten times (10X) magnification. The compound microscope has two systems of lenses for greater magnification, 1) the ocular, or eyepiece lens that one looks into and 2) the objective lens, or the lens closest to the object. Before purchasing or using a microscope, it is important to know the functions of each part. Eyepiece Lens: the lens at the top that you look through. They are usually 10X or 15X power. Tube: Connects the eyepiece to the objective lenses Arm: Supports the tube and connects it to the base. It is used along with the base to carry the microscope Base: The bottom of the microscope, used for support Illuminator: A steady light source (110 volts) used in place of a mirror. Stage: The flat platform where you place your slides. Stage clips hold the slides in place. Revolving Nosepiece or Turret: This is the part that holds two or more objective lenses and can be rotated to easily change power. Objective Lenses: Usually you will find 3 or 4 objective lenses on a microscope. They almost always consist of 4X, 10X, 40X and 100X powers. When coupled with a 10X (most common) eyepiece lens, we get total magnifications of 40X (4X times 10X), 100X , 400X and 1000X. -

How Do the Lenses in a Microscope Work?
Student Name: _____________________________ Date: _________________ How do the lenses in a microscope work? Compound Light Microscope: A compound light microscope uses light to transmit an image to your eye. Compound deals with the microscope having more than one lens. Microscope is the combination of two words; "micro" meaning small and "scope" meaning view. Early microscopes, like Leeuwenhoek's, were called simple because they only had one lens. Simple scopes work like magnifying glasses that you have seen and/or used. These early microscopes had limitations to the amount of magnification no matter how they were constructed. The creation of the compound microscope by the Janssens helped to advance the field of microbiology light years ahead of where it had been only just a few years earlier. The Janssens added a second lens to magnify the image of the primary (or first) lens. Simple light microscopes of the past could magnify an object to 266X as in the case of Leeuwenhoek's microscope. Modern compound light microscopes, under optimal conditions, can magnify an object from 1000X to 2000X (times) the specimens original diameter. "The Compound Light Microscope." The Compound Light Microscope. Web. 16 Feb. 2017. http://www.cas.miamioh.edu/mbi-ws/microscopes/compoundscope.html Text is available under the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) license. - 1 – Student Name: _____________________________ Date: _________________ Now we will describe how a microscope works in somewhat more detail. The first lens of a microscope is the one closest to the object being examined and, for this reason, is called the objective. -

Image Analysis for Face Recognition
Image Analysis for Face Recognition Xiaoguang Lu Dept. of Computer Science & Engineering Michigan State University, East Lansing, MI, 48824 Email: [email protected] Abstract In recent years face recognition has received substantial attention from both research com- munities and the market, but still remained very challenging in real applications. A lot of face recognition algorithms, along with their modifications, have been developed during the past decades. A number of typical algorithms are presented, being categorized into appearance- based and model-based schemes. For appearance-based methods, three linear subspace analysis schemes are presented, and several non-linear manifold analysis approaches for face recognition are briefly described. The model-based approaches are introduced, including Elastic Bunch Graph matching, Active Appearance Model and 3D Morphable Model methods. A number of face databases available in the public domain and several published performance evaluation results are digested. Future research directions based on the current recognition results are pointed out. 1 Introduction In recent years face recognition has received substantial attention from researchers in biometrics, pattern recognition, and computer vision communities [1][2][3][4]. The machine learning and com- puter graphics communities are also increasingly involved in face recognition. This common interest among researchers working in diverse fields is motivated by our remarkable ability to recognize peo- ple and the fact that human activity is a primary concern both in everyday life and in cyberspace. Besides, there are a large number of commercial, security, and forensic applications requiring the use of face recognition technologies. These applications include automated crowd surveillance, ac- cess control, mugshot identification (e.g., for issuing driver licenses), face reconstruction, design of human computer interface (HCI), multimedia communication (e.g., generation of synthetic faces), 1 and content-based image database management. -

Analysis of Artificial Intelligence Based Image Classification Techniques Dr
Journal of Innovative Image Processing (JIIP) (2020) Vol.02/ No. 01 Pages: 44-54 https://www.irojournals.com/iroiip/ DOI: https://doi.org/10.36548/jiip.2020.1.005 Analysis of Artificial Intelligence based Image Classification Techniques Dr. Subarna Shakya Professor, Department of Electronics and Computer Engineering, Central Campus, Institute of Engineering, Pulchowk, Tribhuvan University. Email: [email protected]. Abstract: Time is an essential resource for everyone wants to save in their life. The development of technology inventions made this possible up to certain limit. Due to life style changes people are purchasing most of their needs on a single shop called super market. As the purchasing item numbers are huge, it consumes lot of time for billing process. The existing billing systems made with bar code reading were able to read the details of certain manufacturing items only. The vegetables and fruits are not coming with a bar code most of the time. Sometimes the seller has to weight the items for fixing barcode before the billing process or the biller has to type the item name manually for billing process. This makes the work double and consumes lot of time. The proposed artificial intelligence based image classification system identifies the vegetables and fruits by seeing through a camera for fast billing process. The proposed system is validated with its accuracy over the existing classifiers Support Vector Machine (SVM), K-Nearest Neighbor (KNN), Random Forest (RF) and Discriminant Analysis (DA). Keywords: Fruit image classification, Automatic billing system, Image classifiers, Computer vision recognition. 1. Introduction Artificial intelligences are given to a machine to work on its task independently without need of any manual guidance. -

Face Recognition on a Smart Image Sensor Using Local Gradients
sensors Article Face Recognition on a Smart Image Sensor Using Local Gradients Wladimir Valenzuela 1 , Javier E. Soto 1, Payman Zarkesh-Ha 2 and Miguel Figueroa 1,* 1 Department of Electrical Engineering, Universidad de Concepción, Concepción 4070386, Chile; [email protected] (W.V.); [email protected] (J.E.S.) 2 Department of Electrical and Computer Engineering (ECE), University of New Mexico, Albuquerque, NM 87131-1070, USA; [email protected] * Correspondence: miguel.fi[email protected] Abstract: In this paper, we present the architecture of a smart imaging sensor (SIS) for face recognition, based on a custom-design smart pixel capable of computing local spatial gradients in the analog domain, and a digital coprocessor that performs image classification. The SIS uses spatial gradients to compute a lightweight version of local binary patterns (LBP), which we term ringed LBP (RLBP). Our face recognition method, which is based on Ahonen’s algorithm, operates in three stages: (1) it extracts local image features using RLBP, (2) it computes a feature vector using RLBP histograms, (3) it projects the vector onto a subspace that maximizes class separation and classifies the image using a nearest neighbor criterion. We designed the smart pixel using the TSMC 0.35 µm mixed- signal CMOS process, and evaluated its performance using postlayout parasitic extraction. We also designed and implemented the digital coprocessor on a Xilinx XC7Z020 field-programmable gate array. The smart pixel achieves a fill factor of 34% on the 0.35 µm process and 76% on a 0.18 µm process with 32 µm × 32 µm pixels. The pixel array operates at up to 556 frames per second.