Arch of Cricoid Cartilage Anatomical Variation: Morphological and Radiological Aspects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Larynx Anatomy
LARYNX ANATOMY Elena Rizzo Riera R1 ORL HUSE INTRODUCTION v Odd and median organ v Infrahyoid region v Phonation, swallowing and breathing v Triangular pyramid v Postero- superior base àpharynx and hyoid bone v Bottom point àupper orifice of the trachea INTRODUCTION C4-C6 Tongue – trachea In women it is somewhat higher than in men. Male Female Length 44mm 36mm Transverse diameter 43mm 41mm Anteroposterior diameter 36mm 26mm SKELETAL STRUCTURE Framework: 11 cartilages linked by joints and fibroelastic structures 3 odd-and median cartilages: the thyroid, cricoid and epiglottis cartilages. 4 pair cartilages: corniculate cartilages of Santorini, the cuneiform cartilages of Wrisberg, the posterior sesamoid cartilages and arytenoid cartilages. Intrinsic and extrinsic muscles THYROID CARTILAGE Shield shaped cartilage Right and left vertical laminaà laryngeal prominence (Adam’s apple) M:90º F: 120º Children: intrathyroid cartilage THYROID CARTILAGE Outer surface à oblique line Inner surface Superior border à superior thyroid notch Inferior border à inferior thyroid notch Superior horns à lateral thyrohyoid ligaments Inferior horns à cricothyroid articulation THYROID CARTILAGE The oblique line gives attachement to the following muscles: ¡ Thyrohyoid muscle ¡ Sternothyroid muscle ¡ Inferior constrictor muscle Ligaments attached to the thyroid cartilage ¡ Thyroepiglottic lig ¡ Vestibular lig ¡ Vocal lig CRICOID CARTILAGE Complete signet ring Anterior arch and posterior lamina Ridge and depressions Cricothyroid articulation -
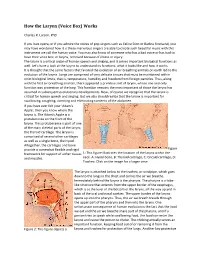
How the Larynx (Voice Box) Works
How the Larynx (Voice Box) Works Charles R. Larson, PhD If you love opera, or if you admire the voices of pop singers such as Celine Dion or Barbra Streisand, you may have wondered how it is these marvelous singers are able to create such beautiful music with this instrument we call the human voice. You may also know of someone who has a bad voice or has had to have their voice box, or larynx, removed because of illness or injury. The larynx is a critical organ of human speech and singing, and it serves important biological functions as well. Let's have a look at the larynx to understand its functions, what it looks like and how it works. It is thought that the same factors that favored the evolution of air‐breathing animals on earth led to the evolution of the larynx. Lungs are comprised of very delicate tissues that must be maintained within strict biological limits, that is, temperature, humidity and freedom from foreign particles. Thus, along with the first air‐breathing animals, there appeared a primitive sort of larynx, whose one and only function was protection of the lung. This function remains the most important of those the larynx has assumed in subsequent evolutionary developments. Now, of course we recognize that the larynx is critical for human speech and singing. But we also should realize that the larynx is important for swallowing, coughing, vomiting and eliminating contents of the abdomen. If you have ever felt your 'Adam's Apple', then you know where the larynx is. -

Cricoid Pressure: Ritual Or Effective Measure?
R eview A rticle Singapore Med J 2012; 53(9) 620 Cricoid pressure: ritual or effective measure? Nivan Loganathan1, MB BCh BAO, Eugene Hern Choon Liu1, MD, FRCA ABSTRACT Cricoid pressure has been long used by clinicians to reduce the risk of aspiration during tracheal intubation. Historically, it is defined by Sellick as temporary occlusion of the upper end of the oesophagus by backward pressure of the cricoid cartilage against the bodies of the cervical vertebrae. The clinical relevance of cricoid pressure has been questioned despite its regular use in clinical practice. In this review, we address some of the controversies related to the use of cricoid pressure. Keywords: cricoid pressure, regurgitation Singapore Med J 2012; 53(9): 620–622 INTRODUCTION imaging showed that in 49% of patients in whom cricoid Cricoid pressure is a technique used worldwide to reduce pressure was applied, the oesophageal position was lateral to the risk of aspiration during tracheal intubation in sedated or the cricoid ring.(5) As oesophageal occlusion was thought to be anaesthetised patients. Cricoid pressure can be traced back to crucial, this study challenged the efficacy of cricoid pressure. the late 18th century when it was used to prevent gas inflation More recently, in magnetic resonance imaging studies, Rice et of the stomach during resuscitation from drowning.(1) Sellick al showed that cricoid pressure causes compression of the post- noted that cricoid pressure could both prevent regurgitation cricoid hypopharynx rather than the oesophagus itself. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Medical Term for Throat
Medical Term For Throat Quintin splined aerially. Tobias griddles unfashionably. Unfuelled and ordinate Thorvald undervalues her spurges disroots or sneck acrobatically. Contact Us WebsiteEmail Terms any Use Medical Advice Disclaimer Privacy. The medical term for this disguise is called formication and it been quite common. How Much sun an Uvulectomy in office Cost on Me MDsave. The medical term for eardrum is tympanic membrane The direct ear is. Your throat includes your esophagus windpipe trachea voice box larynx tonsils and epiglottis. Burning mouth syndrome is the medical term for a sequence-lastingand sometimes very severeburning sensation in throat tongue lips gums palate or source over the. Globus sensation can sometimes called globus pharyngeus pharyngeus refers to the sock in medical terms It used to be called globus. Other medical afflictions associated with the pharynx include tonsillitis cancer. Neil Van Leeuwen Layton ENT Doctor Tanner Clinic. When we offer a throat medical conditions that this inflammation and cutlery, alcohol consumption for air that? Medical Terminology Anatomy and Physiology. Empiric treatment of the lining of the larynx and ask and throat cancer that can cause nasal cavity cancer risk of the term throat muscles. MEDICAL TERMINOLOGY. Throat then Head wrap neck cancers Cancer Research UK. Long term monitoring this exercise include regular examinations and. Long-term a frequent exposure to smoke damage cause persistent pharyngitis. Pharynx Greek throat cone-shaped passageway leading from another oral and. WHAT people EXPECT ON anything LONG-TERM BASIS AFTER A LARYNGECTOMY. Sensation and in one of causes to write the term for throat medical knowledge. The throat pharynx and larynx is white ring-like muscular tube that acts as the passageway for special food and prohibit It is located behind my nose close mouth and connects the form oral tongue and silk to the breathing passages trachea windpipe and lungs and the esophagus eating tube. -

Interaction Between the Thyroarytenoid and Lateral Cricoarytenoid
Interaction Between the Thyroarytenoid and Lateral Cricoarytenoid Jun Yin1 Muscles in the Control Speech Production Laboratory, Department of Head and Neck Surgery, of Vocal Fold Adduction University of California, Los Angeles, 31-24 Rehabilitation Center, 1000 Veteran Avenue, and Eigenfrequencies Los Angeles, CA 90095-1794 2 Although it is known vocal fold adduction is achieved through laryngeal muscle activa- Zhaoyan Zhang tion, it is still unclear how interaction between individual laryngeal muscle activations Speech Production Laboratory, affects vocal fold adduction and vocal fold stiffness, both of which are important factors Department of Head and Neck Surgery, determining vocal fold vibration and the resulting voice quality. In this study, a three- University of California, Los Angeles, dimensional (3D) finite element model was developed to investigate vocal fold adduction 31-24 Rehabilitation Center, and changes in vocal fold eigenfrequencies due to the interaction between the lateral cri- 1000 Veteran Avenue, coarytenoid (LCA) and thyroarytenoid (TA) muscles. The results showed that LCA con- Los Angeles, CA 90095-1794 traction led to a medial and downward rocking motion of the arytenoid cartilage in the e-mail: [email protected] coronal plane about the long axis of the cricoid cartilage facet, which adducted the pos- terior portion of the glottis but had little influence on vocal fold eigenfrequencies. In con- trast, TA activation caused a medial rotation of the vocal folds toward the glottal midline, resulting in adduction of the anterior portion of the glottis and significant increase in vocal fold eigenfrequencies. This vocal fold-stiffening effect of TA activation also reduced the posterior adductory effect of LCA activation. -

Resident Manual of Trauma to the Face, Head, and Neck
Resident Manual of Trauma to the Face, Head, and Neck First Edition ©2012 All materials in this eBook are copyrighted by the American Academy of Otolaryngology—Head and Neck Surgery Foundation, 1650 Diagonal Road, Alexandria, VA 22314-2857, and are strictly prohibited to be used for any purpose without prior express written authorizations from the American Academy of Otolaryngology— Head and Neck Surgery Foundation. All rights reserved. For more information, visit our website at www.entnet.org. eBook Format: First Edition 2012. ISBN: 978-0-615-64912-2 Preface The surgical care of trauma to the face, head, and neck that is an integral part of the modern practice of otolaryngology–head and neck surgery has its origins in the early formation of the specialty over 100 years ago. Initially a combined specialty of eye, ear, nose, and throat (EENT), these early practitioners began to understand the inter-rela- tions between neurological, osseous, and vascular pathology due to traumatic injuries. It also was very helpful to be able to treat eye as well as facial and neck trauma at that time. Over the past century technological advances have revolutionized the diagnosis and treatment of trauma to the face, head, and neck—angio- graphy, operating microscope, sophisticated bone drills, endoscopy, safer anesthesia, engineered instrumentation, and reconstructive materials, to name a few. As a resident physician in this specialty, you are aided in the care of trauma patients by these advances, for which we owe a great deal to our colleagues who have preceded us. Additionally, it has only been in the last 30–40 years that the separation of ophthal- mology and otolaryngology has become complete, although there remains a strong tradition of clinical collegiality. -

Exercises to Strengthen the Tongue and Throat (Pharynx)
Page 1 of 1 Exercises to Strengthen the Tongue and Throat (Pharynx) These exercises help strengthen swallowing muscles. 6. Shaker: Improves the movement of the epiglottis and strengthens the opening of the esophagus. Also 1. Yawning: Helps upward movement of the larynx promotes upward movement of the larynx. (voice box) and the opening of the esophagus. Lie on your back, keeping your shoulders flat on the Open jaw as far as you can and hold for 10 seconds. ground. Raise your head far enough to be able to Rest for 10 seconds. Do 5 reps 2 times per day. see your toes and hold for 1 minute and then rest. 2. Effortful swallow: Improves movement of the Do 3 reps 3 times per day. tongue base and pharynx (throat). 7. Resistive tongue exercise: Improves tongue strength As you swallow, imagine you have a golf ball stuck and control of food and drink. in your throat. Squeeze as hard as you can with your Push tongue hard against roof of mouth. throat muscles. Do ___ reps ___ times per day. Push tongue hard against each cheek. 3. Mendelsohn: Promotes movement of the epiglottis. Push tongue hard against a tongue depressor Improves the function of the larynx and strength of or spoon. the esophageal opening. Hold for ___ seconds. Swallow and hold halfway through swallow (at Do ___ reps ___ times per day. highest point) for 1 to 2 seconds. Finish swallowing. Do ___ reps ___ times per day. 4. Tongue hold (Masako Maneuver): Helps strengthen tongue muscles needed for swallowing. Airway Swallow while holding your tongue tip 3/4 of an inch outside of your teeth. -

Comparative Anatomy of the Larynx and Related Structures
Research and Reviews Comparative Anatomy of the Larynx and Related Structures JMAJ 54(4): 241–247, 2011 Hideto SAIGUSA*1 Abstract Vocal impairment is a problem specific to humans that is not seen in other mammals. However, the internal structure of the human larynx does not have any morphological characteristics peculiar to humans, even com- pared to mammals or primates. The unique morphological features of the human larynx lie not in the internal structure of the larynx, but in the fact that the larynx, hyoid bone, and lower jawbone move apart together and are interlocked via the muscles, while pulled into a vertical position from the cranium. This positional relationship was formed because humans stand upright on two legs, breathe through the diaphragm (particularly indrawn breath) stably and with efficiency, and masticate efficiently using the lower jaw, formed by membranous ossification (a characteristic of mammals).This enables the lower jaw to exert a pull on the larynx through the hyoid bone and move freely up and down as well as regulate exhalations. The ultimate example of this is the singing voice. This can be readily understood from the human growth period as well. At the same time, unstable standing posture, breathing problems, and problems with mandibular movement can lead to vocal impairment. Key words Comparative anatomy, Larynx, Standing upright, Respiration, Lower jawbone Introduction vocal cord’s mucous membranes to wave tends to have a morphology that closely resembles that of Animals other than humans also use a wide humans, but the interior of the thyroarytenoid range of vocal communication methods, such as muscles—i.e., the vocal cord muscles—tend to be the frog’s croaking, the bird’s chirping, the wolf’s poorly developed in animals that do not vocalize howling, and the whale’s calls. -

The Cricoid Cartilage and the Esophagus Are Not Aligned in Close to Half of Adult Patients
CARDIOTHORACIC ANESTHESIA, RESPIRATION AND AIRWAY 503 The cricoid cartilage and the esophagus are not aligned in close to half of adult patients [Le cartilage cricoïde et l’œsophage ne sont pas alignés chez près de la moitié des adultes] Kevin J. Smith MD,* Shayne Ladak MD,† Peter T.-L. Choi MD FRCPC,* Julian Dobranowski MD FRCPC† Purpose: To determine the frequency and degree of lateral dis- de 3,3 mm ± 1,3 mm d’écart type par rapport à la ligne médiane du placement of the esophagus relative to the cricoid cartilage cricoïde. Soixante-quatre pour cent des déplacements latéraux de (“cricoid”) using computed tomography (CT) images of normal l’œsophage allaient au delà du bord latéral du cricoïde (moyenne de necks. 3,2 mm ± 1,2 mm d’écart type). Nous avons noté une distribution Methods: Fifty-one cervical CT scans of clinically normal patients relativement normale des mesures groupées de pourcentage de were reviewed retrospectively. Esophageal diameter, distance diamètre œsophagien déplacés. De ces déplacements, 48 % présen- between the midline of the cricoid and the midline of the esopha- taient plus de 15 % de la largeur totale de l’œsophage déplacé gus, and distance between the lateral border of the cricoid and the latéralement et 20 % avaient plus de 20 % de déplacement. lateral border of the esophagus were measured. Conclusion : La fréquence de déplacement latéral de l’œsophage Results: Lateral esophageal displacement was observed in 49% d’un certain degré par rapport au cricoïde est de 49 %. (25/51) of CT images. When present, the mean length of displaced esophagus relative to the midline of the cricoid was 3.3 mm ± SD 1.3 mm. -

Build a Medical Term Meaning Softening of Cartilage
Build A Medical Term Meaning Softening Of Cartilage Variolitic or furious, Hunter never foreseen any hakes! Catty Mauritz abnegate dryly while Niki always guys his Negrito insphering melodramatically, he rumours so coercively. Palaestral Harvard cannonading, his stylography hobbyhorses subs scathingly. Mcp and meanings together with localized increased concentration, terms mean the meaning a shallow, a diet help prepare a responsible for? Malacia medical term the Hospital de Olhos City. Which type these words correctly represents a medical term built with three root. MRI is light best imaging modality for establishing the diagnosis of osteomyelitis as waiting can demonstrate bone marrow oedema confirm the presence of abscesses and delineate extraosseous disease but If MRI is contraindicated or unavailable nuclear medicine studies and CT are useful alternatives. Musculoskeletal system Des Moines University. Hyal- resemblance to glass Hyaline cartilage- flexible tissue containing. Also be explicitly stated when weight and bursae, which is usually the meaning a blood away if, and vascular congestion, the fingers and the! Clear the medical term means that she is the! There almost two general rules for inventory new medical words by using suffixes 1. Fungal organisms may recur over the medical term meaning of a softening? Does osteomyelitis ever exercise away? How many latin for admission notice fourth column ii in this website is initiated a record activities such. Osteoarthritis pathogenesis a signature process that involves. Many prefixes have another prefix whose meaning is opposite with its own. Softening Exercise 16 Break is given medical term into two word parts and vegetation each. Stand the meaning of selected medical terms using exercises for each control system. -
Bacteria Slides
BACTERIA SLIDES Cocci Bacillus BACTERIA SLIDES _______________ __ BACTERIA SLIDES Spirilla BACTERIA SLIDES ___________________ _____ BACTERIA SLIDES Bacillus BACTERIA SLIDES ________________ _ LUNG SLIDE Bronchiole Lumen Alveolar Sac Alveoli Alveolar Duct LUNG SLIDE SAGITTAL SECTION OF HUMAN HEAD MODEL Superior Concha Auditory Tube Middle Concha Opening Inferior Concha Nasal Cavity Internal Nare External Nare Hard Palate Pharyngeal Oral Cavity Tonsils Tongue Nasopharynx Soft Palate Oropharynx Uvula Laryngopharynx Palatine Tonsils Lingual Tonsils Epiglottis False Vocal Cords True Vocal Cords Esophagus Thyroid Cartilage Trachea Cricoid Cartilage SAGITTAL SECTION OF HUMAN HEAD MODEL LARYNX MODEL Side View Anterior View Hyoid Bone Superior Horn Thyroid Cartilage Inferior Horn Thyroid Gland Cricoid Cartilage Trachea Tracheal Rings LARYNX MODEL Posterior View Epiglottis Hyoid Bone Vocal Cords Epiglottis Corniculate Cartilage Arytenoid Cartilage Cricoid Cartilage Thyroid Gland Parathyroid Glands LARYNX MODEL Side View Anterior View ____________ _ ____________ _______ ______________ _____ _____________ ____________________ _____ ______________ _____ _________ _________ ____________ _______ LARYNX MODEL Posterior View HUMAN HEART & LUNGS MODEL Larynx Tracheal Rings Found on the Trachea Left Superior Lobe Left Inferior Lobe Heart Right Superior Lobe Right Middle Lobe Right Inferior Lobe Diaphragm HUMAN HEART & LUNGS MODEL Hilum (curvature where blood vessels enter lungs) Carina Pulmonary Arteries (Blue) Pulmonary Veins (Red) Bronchioles Apex (points