Diltiazem Hydrochloride Injection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurontin (Gabapentin)
Texas Prior Authorization Program Clinical Criteria Drug/Drug Class Gabapentin Clinical Criteria Information Included in this Document Neurontin (gabapentin) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. Gralise (gabapentin Extended Release) • Drugs requiring prior authorization: the list of drugs requiring prior authorization for this clinical criteria • Prior authorization criteria logic: a description of how the prior authorization request will be evaluated against the clinical criteria rules • Logic diagram: a visual depiction of the clinical criteria logic • Supporting tables: a collection of information associated with the steps within the criteria (diagnosis codes, procedure codes, and therapy codes); provided when applicable • References: clinical publications and sources relevant to this clinical criteria Note: Click the hyperlink to navigate directly to that section. March 29, 2019 Copyright © 2019 Health Information Designs, LLC 1 Horizant -

Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter And
J Am Board Fam Med: first published as 10.3122/jabfm.2011.01.080096 on 5 January 2011. Downloaded from CLINICAL REVIEW Safety and Efficacy of Ibutilide in Cardioversion of Atrial Flutter and Fibrillation Madhuri Nair, MD, Lekha K. George, MD, and Santhosh K. G. Koshy, MD This article reviews the safety and efficacy of ibutilide for use in patients with atrial fibrillation and flut- ter. Ibutilide, a class III antiarrhythmic agent, is primarily used for conversion of atrial flutter and fi- brillation and is a good alternative to electrical cardioversion. Ibutilide has a conversion rate of up to 75% to 80% in recent-onset atrial fibrillation and flutter; the conversion rate is higher for atrial flutter than for atrial fibrillation. It is also safe in the conversion of chronic atrial fibrillation/flutter among patients receiving oral amiodarone therapy. Ibutilide pretreatment facilitates transthoracic defibrilla- tion and decreases the energy requirement of electrical cardioversion by both monophasic and biphasic shocks. Pretreatment with ibutilide before electrical defibrillation has a conversion rate of 100% com- pared with 72% with no pretreatment. Ibutilide is also safe and efficient in the treatment of atrial fibril- lation in patients who have had cardiac surgery, and in accessory pathway–mediated atrial fibrillation where the conversion rate of ibutilide is as high as 95%. There is up to a 4% risk of torsade de pointes and a 4.9% risk of monomorphic ventricular tachycardia. Hence, close monitoring in an intensive care unit setting is warranted during and at least for 4 hours after drug infusion. The anticoagulation strat- egy is the same as for any other mode of cardioversion.(J Am Board Fam Med 2011;24:86–92.) Keywords: Antiarrhythmics, Arrhythmia, Atrial Fibrillation, Cardiovascular Disorders, Cardioversion, Drug Ther- copyright. -

Supplementary Materials
Supplementary Materials Table S1. The significant drug pairs in potential DDIs examined by the two databases. Micromedex Drugs.com List of drugs paired PK-PD Mechanism details 1. Amiodarone— PD Additive QT-interval prolongation Dronedarone 2. Amiodarone— PK CYP3A inhibition by Ketoconazole Ketoconazole 3. Ciprofloxacin— PD Additive QT-interval prolongation Dronedarone 4. Cyclosporine— PK CYP3A inhibition by Cyclosporine Dronedarone 5. Dronedarone— PK CYP3A inhibition by Erythromycin Erythromycin 6. Dronedarone— PD Additive QT-interval prolongation Flecainide 7. Dronedarone— PK CYP3A4 inhibition by Itraconazole Itraconazole 8. Dronedarone— PK Contraindication Major CYP3A inhibition by Ketoconazole Ketoconazole 9. Dronedarone— PD Additive QT-interval prolongation Procainamide PD 10. Dronedarone—Sotalol Additive QT-interval prolongation 11. Felodipine— PK CYP3A inhibition by Itraconazole Itraconazole 12. Felodipine— PK CYP3A inhibition by Ketoconazole Ketoconazole 13. Itraconazole— PK CYP3A inhibition by Itraconazole Nisoldipine 14. Ketoconazole— PK CYP3A inhibition by Ketoconazole Nisoldipine 15. Praziquantel— PK CYP induction by Rifampin Rifampin PD 1. Amikacin—Furosemide Additive or synergistic toxicity 2. Aminophylline— Decreased clearance of PK Ciprofloxacin Theophylline by Ciprofloxacin 3. Aminophylline— PK Decreased hepatic metabolism Mexiletine 4. Amiodarone— PD Additive effects on QT interval Ciprofloxacin 5. Amiodarone—Digoxin PK P-glycoprotein inhibition by Amiodarone 6. Amiodarone— PD, PK Major Major Additive effects on QT Erythromycin prolongation, CYP3A inhibition by Erythromycin 7. Amiodarone— PD, PK Flecainide Antiarrhythmic inhibition by Amiodarone, CYP2D inhibition by Amiodarone 8. Amiodarone— PK CYP3A inhibition by Itraconazole Itraconazole 9. Amiodarone— PD Antiarrhythmic inhibition by Procainamide Amiodarone 10. Amiodarone— PK CYP induction by Rifampin Rifampin PD Additive effects on refractory 11. Amiodarone—Sotalol potential 12. Amiodarone— PK CYP3A inhibition by Verapamil Verapamil 13. -

Sharon R. Roseman, MD, FACP Practice Limited to Gastroenterology
Sharon R. Roseman, MD, FACP Practice Limited to Gastroenterology 701 Broad Street, Suite 411 Sewickley, PA 15143 (412) 749-7160 Fax: (412) 749-7388 http://www.heritagevalley.org/sharonrosemanmd Patient Drug Education for Diltiazem / Nifedipine Ointment Diltiazem/Nifedipine ointment is used to help heal anal fissures. The ointment relaxes the smooth muscle around the anus and promotes blood flow which helps heal the fissure (tear). The ointment reduces anal canal pressure, which diminishes pain and spasm. We use a diluted concentration of Diltiazem/Nifedipine compared to what is typically used for heart patients, and this is why you need to obtain the medication from a pharmacy which will compound your prescription. It is also prescribed to treat anal sphincter spasm, painful hemorrhoids and pelvic floor spasm. The Diltiazem/Nifedipine ointment should be applied 3 times per day, or as directed. A pea-sized drop should be placed on the tip of your finger and then gently placed inside the anus. The finger should be inserted 1/3 – 1/2 its length and may be covered with a plastic glove or finger cot. You may use Vaseline ® to help coat the finger or dilute the ointment. (If you are unable or hesitant to use your finger to administer the ointment TELL U S and we will order you a suppository to use as an “applicator”.) If you are advised to mix the Diltiazem/Nifedipine with steroid ointment, limit the steroids to one to two weeks. The first few applications should be taken lying down, as mild light- headedness or a brief headache may occur. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -
Keeping up with the Pace of Antiarrhythmic Drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J
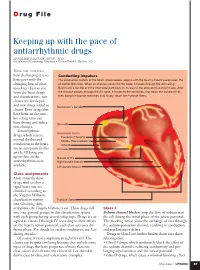
D rug File Keeping up with the pace of antiarrhythmic drugs ANNMARIE PALATNIK, APN,BC, MSN Coordinator of Continuing Education • Virtua Health • Marlton, N.J. HAVE YOU NOTICED how challenging it is to Conducting impulses keep pace with the The conduction system of the heart, shown below, begins with the heart’s natural pacemaker, the changing beat of phar- sinoatrial (SA) node. When an impulse leaves the SA node, it travels through the atria along macology? Just as you Bachmann’s bundle and the internodal pathways on its way to the atrioventricular (AV) node. After learn the latest drugs the impulse passes through the AV node, it travels to the ventricles, first down the bundle of His, and classifications, new then along the bundle branches and, finally, down the Purkinje fibers. classes are developed and new drugs added to Bachmann’s bundle classes. Even drugs that have been on the mar- ket a long time can have dosing and indica- SA node tion changes. Antiarrhythmic Internodal tracts drugs, which restore Posterior (Thorel’s) normal rhythm and Middle (Wenckebach’s) conduction to the heart, Anterior are no exception. In this AV node article, I’ll bring you up-to-date on the Bundle of His antiarrhythmics now Right bundle branch available. Left bundle branch Class assignments Most antiarrhythmic drugs used to slow a rapid heart rate are classified according to the Vaughn Williams classification system Purkinje fibers (see Classifying Anti- arrhythmics the Vaughn Williams way). These drugs fall Class I into four general groups in this classification system Sodium channel blockers stop the flow of sodium into with each group having several subgroups. -

Anaesthetic Implications of Calcium Channel Blockers
436 Anaesthetic implications of calcium channel Leonard C. Jenkins aA MD CM FRCPC blockers Peter J. Scoates a sc MD FRCPC CONTENTS The object of this review is to emphasize the anaesthetic implications of calcium channel block- Physiology - calcium/calcium channel blockers Uses of calcium channel blockers ers for the practising anaesthetist. These drugs have Traditional played an expanding role in therapeutics since their Angina pectoris introduction and thus anaesthetists can expect to see Arrhythmias increasing numbers of patients presenting for anaes- Hypertension thesia who are being treated with calcium channel Newer and investigational Cardiac blockers. Other reviews have emphasized the basic - Hypertrophic cardiomyopathy pharmacology of calcium channel blockers. 1-7 - Cold cardioplegia - Pulmonary hypertension Physiology - calcium/calcium channel blockers Actions on platelets Calcium plays an important role in many physio- Asthma Obstetrics logical processes, such as blood coagulation, en- - Premature labor zyme systems, muscle contraction, bone metabo- - Pre-eclampsia lism, synaptic transmission, and cell membrane Achalasia and oesophageal spasm excitability. Especially important is the role of Increased intraocular pressure therapy calcium in myocardial contractility and conduction Protective effect on kidney after radiocontrast Cerebral vasospasm as well as in vascular smooth muscle reactivity. 7 Induced hypotensive anaesthesia Thus, it can be anticipated that any drug interfering Drag interactions with calcium channel blockers with the action of calcium could have widespread With anaesthetic agents effects. Inhalation agents In order to understand the importance of calcium - Effect on haemodynamics - Effect on MAC in cellular excitation, it is necessary to review some Neuromuscular blockers membrane physiology. Cell membranes are pri- Effects on epinephrine-induced arrhythmias marily phospholipids arranged in a bilayer. -

Atrial Fibrillation in the Surgical Patient
DISCLAIMER: These guidelines were prepared by the Department of Surgical Education, Orlando Regional Medical Center. They are intended to serve as a general statement regarding appropriate patient care practices based upon the available medical literature and clinical expertise at the time of development. They should not be considered to be accepted protocol or policy, nor are intended to replace clinical judgment or dictate care of individual patients. NEW ONSET ATRIAL FIBRILLATION IN THE SURGICAL PATIENT SUMMARY Atrial fibrillation is a common postoperative arrhythmia and can represent a major source of morbidity and mortality. Treatment of atrial fibrillation is directed at three main objectives: controlling the ventricular response, preventing thromboembolism, and maintaining sinus rhythm. Therapeutic decisions also hinge on patients’ hemodynamic stability. In patients who are hemodynamically unstable, direct current cardioversion is the first line therapy and pharmacotherapy should be used as adjunctive treatment. In patients who are hemodynamically stable, pharmacologic treatment including class II (beta-blockers), class III (amiodarone), or class IV (nondihydropyridine calcium channel blockers) agents are viable options. RECOMMENDATIONS Level 1 Beta-blockade (esmolol or metoprolol), nondihydropyridine calcium channel blockers (diltiazem or verapamil), and amiodarone are pharmacologic options to manage new onset atrial fibrillation (see table for dosing). Beta-blockers are the first line therapy for postoperative atrial fibrillation to achieve rapid ventricular rate control and conversion to sinus rhythm. Diltiazem is second line rate control agent when beta-blocker therapy has failed. Both therapies should be avoided in hypotensive patients. Amiodarone can provide both rate and rhythm control and is an alternative therapy to beta-blockade for postoperative atrial fibrillation especially when the patient is hemodynamically unstable or has a known ejection fraction of < 40%. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational -

Calcium Channel Blockers
Calcium Channel Blockers Summary In general, calcium channel blockers (CCBs) are used most often for the management of hypertension and angina. There are 2 classes of CCBs: the dihydropyridines (DHPs), which have greater selectivity for vascular smooth muscle cells than for cardiac myocytes, and the non-DHPs, which have greater selectivity for cardiac myocytes and are used for cardiac arrhythmias. The DHPs cause peripheral edema, headaches, and postural hypotension most commonly, all of which are due to the peripheral vasodilatory effects of the drugs in this class of CCBs. The non-DHPs are negative inotropes and chronotropes; they can cause bradycardia and depress AV node conduction, increasing the risk of heart failure exacerbation, bradycardia, and AV block. Clevidipine is a DHP calcium channel blocker administered via continuous IV infusion and used for rapid blood pressure reductions. All CCBs are substrates of CYP3A4, but both diltiazem and verapamil are also inhibitors of 3A4 and have an increased risk of drug interactions. Verapamil also inhibits CYP2C9, CYP2C19, and CYP1A2. Pharmacology CCBs selectively inhibit the voltage-gated L-type calcium channels on cardiac myocytes, vascular smooth muscle cells, and cells within the sinoatrial (SA) and atrioventricular (AV) nodes, preventing influx of extracellular calcium. CCBs act by either deforming the channels, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the major cellular calcium store, the endoplasmic reticulum. Calcium influx via these channels serves for excitation-contraction coupling and electrical discharge in the heart and vasculature. A decrease in intracellular calcium will result in inhibition of the contractile process of the myocardial smooth muscle cells, resulting in dilation of the coronary and peripheral arterial vasculature. -

Oral Calcium Channel Blocker Comparison
Oral Calcium Channel Blocker Comparison Various calcium channel blockers (CCBs) have been periodically shorted. Below is a table of dosing comparisons. General notes: No dose equivalencies among the CCBs have been established; estimate an approximate dose using the dosing ranges. The contraindications and adverse effects of non-dihydropyridine (DHP) CCBs (diltiazem and verapamil) are quite different from DHP CCBs (amlodipine, felodipine, nifedipine). Consider staying with the same type of CCB if possible unless other considerations warrant changing types. Be sure to check for drug interactions if switching agents. Calcium Channel Blocker Comparisons1,2 Doses CCB Contraindications Hypertension Stable angina DHP Adverse Effects: pedal edema, flushing, palpitations, headache Nifedipine MR 30-60 mg up to 90 mg daily 2.5-5 mg to 10 mg Amlodipine 5-10 mg daily daily severe aortic stenosis 2.5-10 mg to May be useful but Felodipine 20 mg daily not indicated Non-DHP Adverse Effects: angina, heart failure; constipation, especially with verapamil 120-240 mg post myocardial infarction with 120-180 mg to 360 Diltiazem MR to 360 mg ejection fraction (EF) <40% mg daily daily 2nd or 3rd degree AV block, or sick sinus syndrome (unless functioning ventricular pacemaker) atrial flutter/atrial fibrillation and accessory bypass tract (e.g. Wolff- 80-240 mg Parkinson-White syndrome, Lown- 180 mg to 480 mg once daily to Ganong-Levine syndrome) Verapamil MR daily in one or two 180-240 mg combination with ivabradine doses BID Verapamil extreme bradycardia severe heart failure and or EF<40% combination with drugs that affect cardiac conduction CCB= calcium channel blocker; DHP= dihydropyridine; MR=modified release such as XL, CD, SR, etc. -

Procainamide in the Treatment of Myotonia in Myotonic Dystrophy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.45.5.461 on 1 May 1982. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry 1982;45:461-463 Short report A comparative study of disopyramide and procainamide in the treatment of myotonia in myotonic dystrophy MICHAEL FINLAY From the Department ofNeurology, Royal Hallamshire Hospital, Sheffield SUMMARY Ten patients with myotonic dystrophy were allocated at random to treatment with disopyramide and procainamide in a cross-over trial. Disopyramide was found to be at least as effective as procainamide in the relief of myotonia; and two patients who could not tolerate procainamide both tolerated disopyramide. Myotonic dystrophy was first described by Steinert' trophy for between 4 and 21 years were included in the women and the clinical features have been reviewed by trial. There were seven men and three aged be- Protected by copyright. Thomasen2 and others. There have been many tween 31 and 59 years. All complained of weakness of their attempts to elucidate the underlying pathophysi- hands and the majority had noticed difficulty in relaxation of grip. Eight had also experienced impairment of gait. All ology. The basic abnormality has been ascribed to exhibited bilateral ptosis, weakness and wasting of the hypersensitivity of the muscle membrane,3 increased masseters, temporalis, facial and stemomastoid muscles membrane fluidity4 and impairment of normal and wasting and weakness of the forearms and hands. The neurotropic influences exerted by motor neurones seven men had similar distal wasting and weakness of the on muscle fibres.5 6 Quinine, steroids and pro- legs but in two women the lower limbs were symptomati- cainamide have all been used for treatment of the cally normal; in the other woman, there was proximal myotonia with variable success.