C:\Documents and Settings\Megan Kirch\My Documents\3Rd Year\1 Obgyn\Blueprints\01 Pregnancy and Prenatal Care.Htm
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NEW ZEALAND OBSTETRIC ULTRASOUND GUIDELINES 2019 Iii
New Zealand Obstetric Ultrasound Guidelines 2019 Released 2019 health.govt.nz Acknowledgements The Ministry of Health appreciates the time, expertise and commitment of those involved in developing the New Zealand Obstetric Ultrasound Guidelines. Content within these guidelines is derived and modified from Canterbury District Health Board’s obstetric imaging guidelines with permission. All scan images were kindly provided by members of the clinical working group. Members of the clinical working group Dr Rachael McEwing, Radiologist (Chair) Dr David Perry, Paediatric and Obstetric Radiologist Gill Waterhouse, Sonographer Dr Pippa Kyle, Consultant Obstetrician and Maternal Fetal Medicine Specialist Rex de Ryke, Charge Sonographer Peer reviewers Dr Jay Marlow, Maternal Fetal Medicine Specialist Martin Necas, Clinical Specialist Sonographer Dr Rachel Belsham, Radiologist Dr Tom Gentles, Paediatric Cardiologist Citation: Ministry of Health. 2019. New Zealand Obstetric Ultrasound Guidelines. Wellington: Ministry of Health. Published in December 2019 by the Ministry of Health PO Box 5013, Wellington 6140, New Zealand ISBN 978-1-98-859749-2 (online) HP 7284 This document is available at health.govt.nz This work is licensed under the Creative Commons Attribution 4.0 International licence. In essence, you are free to: share ie, copy and redistribute the material in any medium or format; adapt ie, remix, transform and build upon the material. You must give appropriate credit, provide a link to the licence and indicate if changes were made. Abbreviations -

Original Article Comparison of Maternal and Fetal Outcomes of IVF and Spontaneously Conceived Twin Pregnancies: Three Year Experience of a Tertiary Hospital
Int J Clin Exp Med 2015;8(4):6272-6276 www.ijcem.com /ISSN:1940-5901/IJCEM0006677 Original Article Comparison of maternal and fetal outcomes of IVF and spontaneously conceived twin pregnancies: three year experience of a tertiary hospital Ahmet Göçmen1, Şirin Güven2, Simge Bağci1, Yasemin Çekmez1, Fatih Şanlıkan1 1Department of Obstetrics and Gynecology, Ümraniye Medical and Research Hospital, İstanbul, Turkey; 2Depart- ment of Neonatology, Ümraniye Medical and Research Hospital, İstanbul, Turkey Received February 3, 2015; Accepted February 18, 2015; Epub April 15, 2015; Published April 30, 2015 Abstract: Objectives: The aim of this study was to compare maternal and fetal outcomes of spontaneously con- ceived and in-vitro fertilization (IVF) twin pregnancies that were admitted to our obstetric clinic and delivered be- tween January 1, 2011 to November 1, 2014. Material method: A total of 84 twin pregnancies were enrolled for the study and divided into two groups: group 1 as IVF (n = 19) and group 2 as spontaneously conceived (n = 65) twin pregnancies. Data of neonatal various morbidities needs neonatal intensive care unit (NICU) such as necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), sepsis, retinopathy of prematurity (ROP), and intraventricu- lar hemorrhage (IVH) and maternal morbidities such as preeclampsia, eclampsia, postpartum bleeding, gestational diabetes mellitus(GDM) were collected by hospital records. Results: There were no statistical difference between two groups regarding hypertension related to pregnancy, intrauterine growth retardation, Apgar scores, NICU needs, birth weight and height (P > 0.05). The rate of premature rupture of membranes, maternal age, antenatal anemia and premature birth were detected higher in IVF group when compared with the other group (P < 0.05). -

Pre-Term Pre-Labour Rupture of Membranes and the Role of Amniocentesis
Fetal and Maternal Medicine Review 2010; 21:2 75–88 C Cambridge University Press 2010 doi:10.1017/S096553951000001X First published online 15 March 2010 PRE-TERM PRE-LABOUR RUPTURE OF MEMBRANES AND THE ROLE OF AMNIOCENTESIS 1,2 ANNA P KENYON, 1,2 KHALIL N ABI-NADER AND 2 PRANAV P PANDYA 1Elizabeth Garrett Anderson Institute for Women’s Health, University College London, 86-96 Chenies Mews, London WCIE 6NX. 2Fetal Medicine Unit, University College London Hospitals NHS Foundation Trust, 235 Euston Rd, London NWI 2BU. INTRODUCTION Pre-labour premature rupture of membranes (PPROM) is defined as rupture of membranes more than 1 hour prior to the onset of labour at <37 weeks gestation. PPROM occurs in approximately 3% of pregnancies and is responsible for a third of all preterm births.1 Once membranes are ruptured prolonging the pregnancy has no maternal physical advantage but fetal morbidity and mortality are improved daily at early gestations: 19% of those infants born <25 weeks develop cerebral palsy (CP) and 28% have severe motor disability.2 Those infants born extremely pre term (<28 weeks) cost the public sector £75835 (95% CI £27906–145508) per live birth3 not to mention the emotional cost to the family. To prolong gestation is therefore the suggested goal: however how and why might we delay birth in those at risk? PPROM is one scenario associated with preterm birth and here we discuss the causative mechanisms, sequelae, latency, strategies to prolong gestation (antibiotics) and consider the role of amniocentesis. We will also discuss novel therapies. PATHOPHYSIOLOGY OF MEMBRANE RUPTURE The membranes, which act to protect and isolate the fetus, are composed of two layers. -
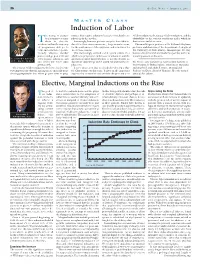
Induction of Labor
36 O B .GYN. NEWS • January 1, 2007 M ASTER C LASS Induction of Labor he timing of parturi- nancies that require induction because of medical com- of labor induction, the timing of labor induction, and the tion remains a conun- plications in the mother. advisability of the various conditions under which in- Tdrum in obstetric Increasingly, however, patients are apt to have labor in- duction can and does occur. medicine in that the majority duced for their own convenience, for personal reasons, This month’s guest professor is Dr. William F. Rayburn, of pregnancies will go to for the convenience of the physician, and sometimes for professor and chairman of the department of ob.gyn. at term and enter labor sponta- all of these reasons. the University of New Mexico, Albuquerque. Dr. Ray- neously, whereas another This increasingly utilized social option ushers in a burn is a maternal and fetal medicine specialist with a na- portion will go post term and whole new perspective on the issue of induction, and the tional reputation in this area. E. ALBERT REECE, often require induction, and question is raised about whether or not the elective in- M.D., PH.D., M.B.A. still others will enter labor duction of labor brings with it added risk and more com- DR. REECE, who specializes in maternal-fetal medicine, is prematurely. plications. Vice President for Medical Affairs, University of Maryland, The concept of labor induction, therefore, has become It is for this reason that we decided to develop a Mas- and the John Z. -

Prenatal Alcohol Consumption Between Conception and Recognition of Pregnancy
ALCOHOLISM:CLINICAL AND EXPERIMENTAL RESEARCH Vol. 41, No. 2 February 2017 Prenatal Alcohol Consumption Between Conception and Recognition of Pregnancy Clare McCormack, Delyse Hutchinson, Lucy Burns, Judy Wilson, Elizabeth Elliott, Steve Allsop, Jake Najman, Sue Jacobs, Larissa Rossen, Craig Olsson, and Richard Mattick Background: Current estimates of the rates of alcohol-exposed pregnancies may underestimate prenatal alcohol exposure if alcohol consumption in early trimester 1, prior to awareness of pregnancy, is not considered. Extant literature describes predictors of alcohol consumption during pregnancy; how- ever, alcohol consumption prior to awareness of pregnancy is a distinct behavior from consumption after becoming aware of pregnancy and thus may be associated with different predictors. The purpose of this study was therefore to examine prevalence and predictors of alcohol consumption by women prior to awareness of their pregnancy, and trajectories of change to alcohol use following pregnancy recognition. Methods: Pregnant women (n = 1,403) were prospectively recruited from general antenatal clinics of 4 public hospitals in Australian metropolitan areas between 2008 and 2013. Women completed detailed interviews about alcohol use before and after recognition of pregnancy. Results: Most women (n = 850, 60.6%) drank alcohol between conception and pregnancy recogni- tion. Binge and heavy drinking were more prevalent than low-level drinking. The proportion of women who drank alcohol reduced to 18.3% (n = 257) after recognition of pregnancy. Of women who drank alcohol, 70.5% ceased drinking, 18.3% reduced consumption, and 11.1% made no reduction following awareness of pregnancy. Socioeconomic status (SES) was the strongest predictor of alcohol use, with drinkers more likely to be of high rather than low SES compared with abstainers (OR = 3.30, p < 0.001). -

Heterotopic Pregnancy and Septic Miscarriage:A Case Report
Journal of Gynecology and Women’s Health ISSN 2474-7602 Case Report J Gynecol Women’s Health Volume 18 Issue 5 - June 2020 Copyright © All rights are reserved by Frédéric Blavier DOI: 10.19080/JGWH.2020.18.556004 Heterotopic Pregnancy and Septic Miscarriage: A Case Report Illustrating Risks of “Medical Shopping”, Mostly for Tricky Diagnoses Frédéric B1,2, Gilles F1, Danah VO1, Noëlie D3 and Leonardo G1 1Department of Obstetrics and Prenatal Medicine, UZ Brussel University Hospital, Belgium 2Department of gynecological surgery, Arnaud de Villeneuve Hospital, France 3Department of Gynecology, UZ Brussel University Hospital, Belgium Submission: May 09, 2020; Published: June 09, 2020 *Corresponding author: Frédéric Blavier, Department of Obstetrics and Prenatal Medicine, UZ Brussel University Hospital, Belgium and Department of gynecological surgery, Arnaud de Villeneuve Hospital, France Abstract Background: Spontaneous heterotopic pregnancy and septic miscarriage are both rare and, each, associated with potential severe morbidity (shock, potential infertility) and mortality. Case presentation: A young and spontaneously pregnant woman presented acute abdomen and spotting with an empty uterus, liquid in for a suspected ectopic pregnancy, were discovered ascites, and pus in the right fallopian tube. After surgery, we observed a multiple organ the Douglas and a right tube mass in ultrasound with positif hCG and moderate inflammation, without fever. During the laparoscopy performed mechanical ventilation, temporary hemodialysis and broad-spectrum antibiotherapy, the patient recovered without neurological or systemic failure (ARDS, DIC, kidneys and liver insufficiencies) with severe inflammation and several collections in the uterine wall. After hysterectomy, abortion diagnosed one week ago in a third hospital also in Brussels. The multiple organ failure, the pus in the right fallopian tube (observed duringsequelae. -

PLANNED OUT-OF-HOSPITAL BIRTH Approved 11/12/15
HEALTH EVIDENCE REVIEW COMMISSION (HERC) COVERAGE GUIDANCE: PLANNED OUT-OF-HOSPITAL BIRTH Approved 11/12/15 HERC COVERAGE GUIDANCE Planned out-of-hospital (OOH) birth is recommended for coverage for women who do not have high- risk coverage exclusion criteria as outlined below (weak recommendation). This coverage recommendation is based on the performance of appropriate risk assessments1 and the OOH birth attendant’s compliance with the consultation and transfer criteria as outlined below. Planned OOH birth is not recommended for coverage for women who have high risk coverage exclusion criteria as outlined below, or when appropriate risk assessments are not performed, or where the attendant does not comply with the consultation and transfer criteria as outlined below (strong recommendation). High-risk coverage exclusion criteria: Complications in a previous pregnancy: Maternal surgical history Cesarean section or other hysterotomy Uterine rupture Retained placenta requiring surgical removal Fourth-degree laceration without satisfactory functional recovery Maternal medical history Pre-eclampsia requiring preterm birth Eclampsia HELLP syndrome Fetal Unexplained stillbirth/neonatal death or previous death related to intrapartum difficulty Baby with neonatal encephalopathy Placental abruption with adverse outcome Complications of current pregnancy: Maternal Induction of labor Prelabor rupture of membranes > 24 hours 1 Pre-existing chronic hypertension; Pregnancy-induced hypertension with diastolic blood pressure greater than -

ABCDE Acronym Blood Transfusion 231 Major Trauma 234 Maternal
Cambridge University Press 978-0-521-26827-1 - Obstetric and Intrapartum Emergencies: A Practical Guide to Management Edwin Chandraharan and Sir Sabaratnam Arulkumaran Index More information Index ABCDE acronym albumin, blood plasma levels 7 arterial blood gas (ABG) 188 blood transfusion 231 allergic anaphylaxis 229 arterio-venous occlusions 166–167 major trauma 234 maternal collapse 12, 130–131 amiadarone, overdose 178 aspiration 10, 246 newborn infant 241 amniocentesis 234 aspirin 26, 180–181 resuscitation 127–131 amniotic fluid embolism 48–51 assisted reproduction 93 abdomen caesarean section 257 asthma 4, 150, 151, 152, 185 examination after trauma 234 massive haemorrhage 33 pain in pregnancy 154–160, 161 maternal collapse 10, 13, 128 atracurium, drug reactions 231 accreta, placenta 250, 252, 255 anaemia, physiological 1, 7 atrial fibrillation 205 ACE inhibitors, overdose 178 anaerobic metabolism 242 automated external defibrillator (AED) 12 acid–base analysis 104 anaesthesia. See general anaesthesia awareness under anaesthesia 215, 217 acidosis 94, 180–181, 186, 242 anal incontinence 138–139 ACTH levels 210 analgesia 11, 100, 218 barbiturates, overdose 178 activated charcoal 177, 180–181 anaphylaxis 11, 227–228, 229–231 behaviour/beliefs, psychiatric activated partial thromboplastin time antacid prophylaxis 217 emergencies 172 (APTT) 19, 21 antenatal screening, DVT 16 benign intracranial hypertension 166 activated protein C 46 antepartum haemorrhage 33, 93–94. benzodiazepines, overdose 178 Addison’s disease 208–209 See also massive -

Review Article Umbilical Cord Hematoma: a Case Report and Review of the Literature
Hindawi Obstetrics and Gynecology International Volume 2018, Article ID 2610980, 6 pages https://doi.org/10.1155/2018/2610980 Review Article Umbilical Cord Hematoma: A Case Report and Review of the Literature Gennaro Scutiero,1 Bernardi Giulia,1 Piergiorgio Iannone ,1 Luigi Nappi,2 Danila Morano,1 and Pantaleo Greco1 1Department of Morphology, Surgery and Experimental Medicine, Section of Obstetrics and Gynecology, Azienda Ospedaliero-Universitaria S. Anna, University of Ferrara, Via Aldo Moro 8, 44121 Cona, Ferrara, Italy 2Department of Medical and Surgical Sciences, Institute of Obstetrics and Gynecology, University of Foggia, Viale L. Pinto, 71100 Foggia, Italy Correspondence should be addressed to Piergiorgio Iannone; [email protected] Received 17 December 2017; Accepted 21 February 2018; Published 26 March 2018 Academic Editor: John J. Moore Copyright © 2018 Gennaro Scutiero et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Objectives. To deepen the knowledge in obstetrics on a very rare pregnancy complication: umbilical cord hematoma. Methods.A review of the case reports described in the last ten years in the literature was conducted in order to evaluate epidemiology, predisposing factors, potential outcomes, prenatal diagnosis, and clinical management. Results. Spontaneous umbilical cord hematoma is a rare complication of pregnancy which represents a serious cause of fetal morbidity and mortality. *ere are many risk factors such as morphologic anomalies, infections, vessel wall abnormalities, iatrogenic causes, and traction or torsion of the cord, but the exact etiology is still unknown. -

15 Complications of Labor and Birth 279
Complications of 15 Labor and Birth CHAPTER CHAPTER http://evolve.elsevier.com/Leifer/maternity Objectives augmentation of labor (a˘wg-me˘n-TA¯ -shu˘n, p. •••) Bishop score (p. •••) On completion and mastery of Chapter 15, the student will be able to do cephalopelvic disproportion (CPD) (se˘f-a˘-lo¯ -PE˘L- v i˘c the following: di˘s-pro¯ -PO˘ R-shu˘n, p. •••) 1. Defi ne key terms listed. cesarean birth (se˘-ZA¯ R-e¯-a˘n, p. •••) 2. Discuss four factors associated with preterm labor. chorioamnionitis (ko¯ -re¯-o¯-a˘m-ne¯-o¯ -NI¯-ti˘s, p. •••) 3. Describe two major nursing assessments of a woman dysfunctional labor (p. •••) ˘ ¯ in preterm labor. dystocia (dis-TO-se¯-a˘, p. •••) episiotomy (e˘-pe¯ z-e¯-O˘ T- o¯ -me¯, p. •••) 4. Explain why tocolytic agents are used in preterm labor. external version (p. •••) 5. Interpret the term premature rupture of membranes. fern test (p. •••) 6. Identify two complications of premature rupture of forceps (p. •••) membranes. hydramnios (hi¯-DRA˘ M-ne¯-o˘s, p. •••) 7. Differentiate between hypotonic and hypertonic uterine hypertonic uterine dysfunction (hi¯-pe˘r-TO˘ N-i˘k U¯ -te˘r-i˘n, dysfunction. p. •••) 8. Name and describe the three different types of breech hypotonic uterine dysfunction (hi¯-po¯-TO˘ N-i˘k, p. •••) presentation. induction of labor (p. •••) ¯ 9. List two potential complications of a breech birth. multifetal pregnancy (mu˘l-te¯-FE-ta˘l, p. •••) nitrazine paper test (NI¯-tra˘-ze¯n, p. •••) 10. Explain the term cephalopelvic disproportion (CPD), and oligohydramnios (p. •••) discuss the nursing management of CPD. -

And Double-Balloon Catheters for Cervical Ripening and Labor Induction
Comparison Between The Ecacy and Safety of The Single- (Love Baby) and Double-Balloon Catheters for Cervical Ripening and Labor Induction Meng Hou Xi'an Jiaotong University https://orcid.org/0000-0002-6510-886X Weihong Wang Xi'an Jiaotong University Medical College First Aliated Hospital Dan Liu Xi'an Jiaotong University Medical College First Aliated Hospital Xuelan Li ( [email protected] ) Xi'an Jiaotong University Medical College First Aliated Hospital Research article Keywords: cervix, balloon catheters, single- and double-balloon catheters, labor induction, cervical ripening Posted Date: July 16th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-37246/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/23 Abstract Background: Induced labor is а progressively common obstetric procedure, Whether the specically designed double-balloon catheter is better than the single-balloon device in terms of ecacy, eciency and safety yet remains controversial. Methods: In our study We have performed a Retrospective study in which 220 patients with immature cervix were admitted for induction of labor either through single cervix balloon catheter (love-baby) (SBC) or double cervix balloon catheter (DBC). The comparison showed that the cervical bishop score was slightly higher for the SBC after removal or expulsion of the balloon. Results:This was a proof that SBC demonstrates slightly better ecacy for cervical ripening with a shorter time from balloon placement to spontaneous vaginal delivery than DBC. No signicant differences in the comparison between SBC and DBC following other parameters like spontaneous vaginal delivery, the initiate uterine contractions rate, the number of patients that needed oxytocin, the balloon spontaneous expulsion rate and others have been detected. -

A Guide to Obstetrical Coding Production of This Document Is Made Possible by Financial Contributions from Health Canada and Provincial and Territorial Governments
ICD-10-CA | CCI A Guide to Obstetrical Coding Production of this document is made possible by financial contributions from Health Canada and provincial and territorial governments. The views expressed herein do not necessarily represent the views of Health Canada or any provincial or territorial government. Unless otherwise indicated, this product uses data provided by Canada’s provinces and territories. All rights reserved. The contents of this publication may be reproduced unaltered, in whole or in part and by any means, solely for non-commercial purposes, provided that the Canadian Institute for Health Information is properly and fully acknowledged as the copyright owner. Any reproduction or use of this publication or its contents for any commercial purpose requires the prior written authorization of the Canadian Institute for Health Information. Reproduction or use that suggests endorsement by, or affiliation with, the Canadian Institute for Health Information is prohibited. For permission or information, please contact CIHI: Canadian Institute for Health Information 495 Richmond Road, Suite 600 Ottawa, Ontario K2A 4H6 Phone: 613-241-7860 Fax: 613-241-8120 www.cihi.ca [email protected] © 2018 Canadian Institute for Health Information Cette publication est aussi disponible en français sous le titre Guide de codification des données en obstétrique. Table of contents About CIHI ................................................................................................................................. 6 Chapter 1: Introduction ..............................................................................................................