Scientific Background
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wo 2010/075090 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 1 July 2010 (01.07.2010) WO 2010/075090 A2 (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C07D 409/14 (2006.01) A61K 31/7028 (2006.01) kind of national protection available): AE, AG, AL, AM, C07D 409/12 (2006.01) A61P 11/06 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, (21) International Application Number: DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US2009/068073 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 December 2009 (15.12.2009) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, (25) Filing Language: English SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, (26) Publication Language: English TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 61/122,478 15 December 2008 (15.12.2008) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (71) Applicant (for all designated States except US): AUS- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, PEX PHARMACEUTICALS, INC. -

Methylxanthines and Neurodegenerative Diseases: an Update
nutrients Review Methylxanthines and Neurodegenerative Diseases: An Update Daniel Janitschke 1,† , Anna A. Lauer 1,†, Cornel M. Bachmann 1, Heike S. Grimm 1, Tobias Hartmann 1,2 and Marcus O. W. Grimm 1,2,* 1 Experimental Neurology, Saarland University, 66421 Homburg/Saar, Germany; [email protected] (D.J.); [email protected] (A.A.L.); [email protected] (C.M.B.); [email protected] (H.S.G.); [email protected] (T.H.) 2 Deutsches Institut für DemenzPrävention (DIDP), Saarland University, 66421 Homburg/Saar, Germany * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Methylxanthines (MTX) are purine derived xanthine derivatives. Whereas naturally occurring methylxanthines like caffeine, theophylline or theobromine are widely consumed in food, several synthetic but also non-synthetic methylxanthines are used as pharmaceuticals, in particular in treating airway constrictions. Besides the well-established bronchoprotective effects, methylxanthines are also known to have anti-inflammatory and anti-oxidative properties, mediate changes in lipid homeostasis and have neuroprotective effects. Known molecular mechanisms include adenosine receptor antagonism, phosphodiesterase inhibition, effects on the cholinergic system, wnt signaling, histone deacetylase activation and gene regulation. By affecting several pathways associated with neurodegenerative diseases via different pleiotropic mechanisms and due to its moderate side effects, intake of methylxanthines have been suggested to be an interesting approach in dealing with neurodegeneration. Especially in the past years, the impact of methylxanthines in neurodegenerative diseases has been extensively studied and several new aspects have been elucidated. In this review Citation: Janitschke, D.; Lauer, A.A.; Bachmann, C.M.; Grimm, H.S.; we summarize the findings of methylxanthines linked to Alzheimer´s disease, Parkinson’s disease Hartmann, T.; Grimm, M.O.W. -

Comparison of Caffeine and D-Amphetamine in Cocaine-Dependent Subjects: Differential Outcomes on Subjective and Cardiovascular Effects, Reward Learning, and Salivary
NIH Public Access Author Manuscript J Addict Res Ther. Author manuscript; available in PMC 2014 November 18. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Addict Res Ther. 2014 ; 5(2): 176–. doi:10.4172/2155-6105.1000176. Comparison of Caffeine and d-amphetamine in Cocaine- Dependent Subjects: Differential Outcomes on Subjective and Cardiovascular Effects, Reward Learning, and Salivary Paraxanthine Scott D Lane1,*, Charles E Green1, Joy M Schmitz1, Nuvan Rathnayaka1, Wendy B Fang2, Sergi Ferré3, and F Gerard Moeller4 1Center for Neurobehavioral Research on Addictions, Department of Psychiatry & Behavioral Sciences, University of Texas Health Science Center – Houston, Houston, TX USA 2Center for Human Toxicology, University of Utah, Salt Lake City, UT, USA 3Integrative Neurobiology, Intramural Research Program, National Institute on Drug Abuse, Baltimore, MD, USA 4Division on Addictions, Department of Psychiatry, Virginia Commonwealth University, Richmond, VA, USA Abstract Due to indirect modulation of dopamine transmission, adenosine receptor antagonists may be useful in either treating cocaine use or improving disrupted cognitive-behavioral functions associated with chronic cocaine use. To compare and contrast the stimulant effects of adenosine antagonism to direct dopamine stimulation, we administered 150 mg and 300 mg caffeine, 20 mg amphetamine, and placebo to cocaine-dependent vs. healthy control subjects, matched on moderate caffeine use. Data were obtained on measures -

(12) Patent Application Publication (10) Pub. No.: US 2011/0142957 A1 VAN KEMPEN (43) Pub
US 2011 O142957A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/0142957 A1 VAN KEMPEN (43) Pub. Date: Jun. 16, 2011 (54) METHOD OF TREATING PARTURIENT Publication Classification PLACENTAL MAMMALS IN ORDER TO (51) Int. Cl. REDUCE MATERNAL AND/OR UTERINE A633/42 (2006.01) EXHAUSTION A633/04 (2006.01) A633/00 (2006.01) (76) Inventor: Theo VAN KEMPEN, Sint Stevens A6II 3/522 (2006.01) Woluwe (BE) A63L/35 (2006.01) (21) Appl. No.: 13/030,422 (52) U.S. Cl. ... 424/601; 424/709: 424/722: 514/263.34; 514/654 (22) Filed: Feb. 18, 2011 (57) ABSTRACT Related U.S. Application Data The present invention relates to a method of facilitating the birth process of placental mammals, especially to a method of (62) Division of application No. 12/063,830, filed on Aug. reducing delays in the birth process and, thereby, complica 4, 2008, now Pat. No. 7,893,070, filed as application tions resulting there from that may negatively affect the health No. PCT/NL2006/050201 on Aug. 14, 2006. and wellbeing of the mother and increase the incidence of stillbirths and/or neonatal mortality. According to the present (60) Provisional application No. 60/707,954, filed on Aug. invention delays in parturition that result from maternal and/ 15, 2005. or uterine exhaustion may be prevented or reduced by the administration of an effective amount of one or more psycho (30) Foreign Application Priority Data motor stimulants to the parturient mammal prior to and/or during parturition. Said psychomotor stimulant is selected Aug. -

The Transcriptional Repressor Orphan Nuclear Receptor TLX Is Responsive to Caffeine and Istradefylline
The transcriptional repressor orphan nuclear receptor TLX is responsive to caffeine and istradefylline Giuseppe Faudonea, Whitney Kilua, Xiaomin Nia,b, Apirat Chaikuada,b, Sridhar Sreeramuluc, Pascal Heitela, Harald Schwalbec, Stefan Knappa,b, Manfred Schubert-Zsilavecza, Jan Heeringd, and Daniel Merka* a Institute of Pharmaceutical Chemistry, Goethe University Frankfurt, Max-von-Laue-Str. 9, D-60438 Frankfurt, Germany b Structural Genomics Consortium, BMLS, Goethe University Frankfurt, Max-von-Laue-Str. 15, D-60438 Frankfurt, Germany c Center for Biomolecular Magnetic Resonance (BMRZ), Institute for Organic Chemistry and Chemical Biology, Goethe University Frankfurt, Max-von-Laue-Str. 7, D-60438 Frankfurt, Germany d Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, Theodor-Stern-Kai 7, D-60596 Frankfurt, Germany * Correspondence to: [email protected] Abstract. The orphan nuclear receptor TLX is expressed almost exclusively in neural stem cells. TLX acts as an essential factor for neural stem cell survival and is hence considered as a promising drug target in neurodegeneration. However, few studies have characterized the roles of TLX due to a lack of ligands and limited functional understanding. Here, we identify caffeine and istradefylline as TLX ligands that counteract the receptor’s intrinsic repressor activity in reporter gene assays and modulate TLX regulated SIRT1 and p21 expression. Mutagenesis of residues lining a cavity within the TLX ligand binding domain altered activity of these ligands suggesting direct interactions with helix 5. Using istradefylline as a tool compound, we observed ligand-sensitive recruitment of the co-repressor SMRT and heterodimerization of TLX with RXR. Both protein-protein complexes evolve as factors that modulate TLX function and suggest an unprecedented role of TLX in directly repressing other nuclear receptors. -

US 2002/0103350 A1 Lyles (43) Pub
US 2002O103350A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0103350 A1 Lyles (43) Pub. Date: Aug. 1, 2002 (54) MATERIALS AND METHODS FOR BINDING Publication Classification NUCLECACDS TO SURFACES (51) Int. CI.7. ... C12O 1/68; CO7H 21/04 (76) Inventor: Mark B. Lyles, San Antonio, TX (US) (52) U.S. Cl. ............................................... 536/23.1; 435/6 Correspondence Address: (57) ABSTRACT Baker Botts L.L.P. Surfaces containing high purity Silica (Silicon dioxide) One Shell Plaza exhibit high loading potential for nucleic acids. 901 Louisiana Houston, TX 77002-4995 (US) Formulations containing nucleic acids and materials which mask the electroStatic interactions between the nucleic acids (21) Appl. No.: 09/910,697 and Surfaces are disclosed. By masking the phosphate charges of the nucleic acids, undesired interactions may be (22) Filed: Jul. 20, 2001 minimized or eliminated, thereby allowing the covalent bonding of the nucleic acids to the Surface to proceed. The use of Such formulations additionally minimizes nonspecific Related U.S. Application Data binding of the nucleic acids to the Surface. Examples of materials to be included in Such formulations include cat (60) Provisional application No. 60/220,096, filed on Jul. ions, Xanthines, heXOSes, purines, arginine, lysine, polyargi 21, 2000. nine, polylysine, and quaternary ammonium Salts. US 2002/0103350 A1 Aug. 1, 2002 MATERIALS AND METHODS FOR BINDING DETAILED DESCRIPTION OF THE NUCLEIC ACIDS TO SURFACES INVENTION 0009. The prior art materials and formulations have been FIELD OF THE INVENTION plagued with two general problems; a) low loading potential 0001. The invention relates to silica Surfaces useful for of the Surfaces, and b) nonspecific binding of nucleic acids binding nucleic acids, and formulations to improve the to the Surface. -

Simultaneous Quantification of Antioxidants Paraxanthine
antioxidants Article Simultaneous Quantification of Antioxidants Paraxanthine and Caffeine in Human Saliva by Electrochemical Sensing for CYP1A2 Phenotyping Rozalia-Maria Anastasiadi 1,*, Federico Berti 2 , Silvia Colomban 3 , Claudio Tavagnacco 2, Luciano Navarini 3 and Marina Resmini 1,* 1 Department of Chemistry, Queen Mary University of London, Mile End Road, London E1 4NS, UK 2 Department of Chemical and Pharmaceutical Sciences, University of Trieste, via L. Giorgieri 1, 34127 Trieste, Italy; [email protected] (F.B.); [email protected] (C.T.) 3 Aromalab, illycaffè S.p.A., Area Science Park, Localita’ Padriciano 99, 34149 Trieste, Italy; [email protected] (S.C.); [email protected] (L.N.) * Correspondence: [email protected] (R.-M.A.); [email protected] (M.R.) Abstract: The enzyme CYP1A2 is responsible for the metabolism of numerous antioxidants in the body, including caffeine, which is transformed into paraxanthine, its main primary metabolite. Both molecules are known for their antioxidant and pro-oxidant characteristics, and the paraxanthine- to-caffeine molar ratio is a widely accepted metric for CYP1A2 phenotyping, to optimize dose– response effects in individual patients. We developed a simple, cheap and fast electrochemical based method for the simultaneous quantification of paraxanthine and caffeine in human saliva, by differ- ential pulse voltammetry, using an anodically pretreated glassy carbon electrode. Cyclic voltammetry experiments revealed for the first time that the oxidation of paraxanthine is diffusion controlled with an irreversible peak at ca. +1.24 V (vs. Ag/AgCl) in a 0.1 M H2SO4 solution, and that the mechanism occurs via the transfer of two electrons and two protons. -

Download Product Insert (PDF)
PRODUCT INFORMATION Paraxanthine Item No. 21068 CAS Registry No.: 611-59-6 Formal Name: 3,7-dihydro-1,7-dimethyl-1H-purine-2,6-dione O Synonyms: 1,7-Dimethylxanthine, NSC 400018 N MF: C7H8N4O2 N FW: 180.2 Purity: ≥98% N N O UV/Vis.: λmax: 269 nm Supplied as: A crystalline solid H Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Paraxanthine is supplied as a crystalline solid. A stock solution may be made by dissolving the paraxanthine in the solvent of choice. Paraxanthine is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of paraxanthine in these solvents is approximately 0.5, 30, and 20 mg/ml, respectively. Paraxanthine is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, paraxanthine should first be dissolved in DMSO and then diluted with the aqueous buffer of choice. Paraxanthine has a solubility of approximately 0.25 mg/ml in a 1:3 solution of DMSO:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Paraxanthine is an inhibitor of phosphodiesterase 9 (PDE9) and an antagonist of adenosine receptors 1,2 A1 and A2 (Kis = 35 and 22 μM, respectively in equine forebrain tissues). It is the main metabolite of caffeine (Item No. 14118) in humans, making up 80% of the three dimethylxanthine metabolites produced by caffeine demethylation.1,3 Paraxanthine increases locomotor activity and counteracts adenosine receptor agonist-induced motor depression in rats not habituated to caffeine.1 At a dose of 30 mg/kg, paraxanthine induces a significant increase in striatal cGMP and extracellular striatal dopamine concentrations in vivo. -

A Complex but Promising Therapeutic Target for Alzheimer's Disease
The Adenosinergic Signaling: A Complex but Promising Therapeutic Target for Alzheimer’s Disease Luc Cellai, Kevin Carvalho, Emilie Faivre, Aude Deleau, Didier Vieau, Luc Buée, David Blum, Céline Mériaux, Victoria Gomez-Murcia To cite this version: Luc Cellai, Kevin Carvalho, Emilie Faivre, Aude Deleau, Didier Vieau, et al.. The Adenosinergic Signaling: A Complex but Promising Therapeutic Target for Alzheimer’s Disease. Frontiers in Neu- roscience, Frontiers, 2018, 12, pp.520. 10.3389/fnins.2018.00520. inserm-01930487 HAL Id: inserm-01930487 https://www.hal.inserm.fr/inserm-01930487 Submitted on 22 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. fnins-12-00520 August 1, 2018 Time: 8:2 # 1 MINI REVIEW published: 03 August 2018 doi: 10.3389/fnins.2018.00520 The Adenosinergic Signaling: A Complex but Promising Therapeutic Target for Alzheimer’s Disease Lucrezia Cellai†, Kevin Carvalho†, Emilie Faivre, Aude Deleau, Didier Vieau, Luc Buée, David Blum*, Céline Mériaux‡ and Victoria Gomez-Murcia‡ Institut National de la Santé et de la Recherche Médicale, CHU Lille, UMR-S 1172-JPArc, LabEx DISTALZ, Université de Lille, Lille, France Alzheimer’s disease (AD) is the most common neurodegenerative disorder in elderly people. -

2021 Equine Prohibited Substances List
2021 Equine Prohibited Substances List . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. LISTED AS SUBSTANCE ACTIVITY BANNED 1-androsterone Anabolic BANNED 3β-Hydroxy-5α-androstan-17-one Anabolic BANNED 4-chlorometatandienone Anabolic BANNED 5α-Androst-2-ene-17one Anabolic BANNED 5α-Androstane-3α, 17α-diol Anabolic BANNED 5α-Androstane-3α, 17β-diol Anabolic BANNED 5α-Androstane-3β, 17α-diol Anabolic BANNED 5α-Androstane-3β, 17β-diol Anabolic BANNED 5β-Androstane-3α, 17β-diol Anabolic BANNED 7α-Hydroxy-DHEA Anabolic BANNED 7β-Hydroxy-DHEA Anabolic BANNED 7-Keto-DHEA Anabolic CONTROLLED 17-Alpha-Hydroxy Progesterone Hormone FEMALES BANNED 17-Alpha-Hydroxy Progesterone Anabolic MALES BANNED 19-Norandrosterone Anabolic BANNED 19-Noretiocholanolone Anabolic BANNED 20-Hydroxyecdysone Anabolic BANNED Δ1-Testosterone Anabolic BANNED Acebutolol Beta blocker BANNED Acefylline Bronchodilator BANNED Acemetacin Non-steroidal anti-inflammatory drug BANNED Acenocoumarol Anticoagulant CONTROLLED Acepromazine Sedative BANNED Acetanilid Analgesic/antipyretic CONTROLLED Acetazolamide Carbonic Anhydrase Inhibitor BANNED Acetohexamide Pancreatic stimulant CONTROLLED Acetominophen (Paracetamol) Analgesic BANNED Acetophenazine Antipsychotic BANNED Acetophenetidin (Phenacetin) Analgesic BANNED Acetylmorphine Narcotic BANNED Adinazolam Anxiolytic BANNED Adiphenine Antispasmodic BANNED Adrafinil Stimulant 1 December 2020, Lausanne, Switzerland 2021 Equine Prohibited Substances List . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). -

A Non-Imaging High Throughput Approach to Chemical Library Screening at the Unmodified Adenosine-A3 Receptor in Living Cells
ORIGINAL RESEARCH published: 13 December 2017 doi: 10.3389/fphar.2017.00908 A Non-imaging High Throughput Approach to Chemical Library Screening at the Unmodified Adenosine-A3 Receptor in Living Cells Maria Augusta Arruda 1, 2, 3†, Leigh A. Stoddart 1, 2†, Karolina Gherbi 1, 2, Stephen J. Briddon 1, 2*, Barrie Kellam 4 and Stephen J. Hill 1, 2* 1 Division of Physiology, Pharmacology and Neuroscience, School of Life Sciences, Medical School, Queen’s Medical Centre, University of Nottingham, Nottingham, United Kingdom, 2 Centre of Membrane Proteins and Receptors, University of Birmingham and University of Nottingham, The Midlands, United Kingdom, 3 Vice-Diretoria de Ensino, Pesquisa e Inovacao, Farmanguinhos, Fiocruz, Rio de Janeiro, Brazil, 4 Centre for Biomolecular Sciences, School of Pharmacy, University of Nottingham, Nottingham, United Kingdom Edited by: Kenneth A. Jacobson, National Institutes of Health (NIH), Recent advances in fluorescent ligand technology have enabled the study of G United States protein-coupled receptors in their native environment without the need for genetic Reviewed by: Vittoria Colotta, modification such as addition of N-terminal fluorescent or bioluminescent tags. Here, University of Florence, Italy we have used a non-imaging plate reader (PHERAstar FS) to monitor the binding Eddy Sotelo, of fluorescent ligands to the human adenosine-A receptor (A AR; CA200645 and Universidade de Santiago de 3 3 Compostela, Spain AV039), stably expressed in CHO-K1 cells. To verify that this method was suitable *Correspondence: for the study of other GPCRs, assays at the human adenosine-A1 receptor, and β1 Stephen J. Briddon and β2 adrenoceptors (β1AR and β2AR; BODIPY-TMR-CGP-12177) were also carried [email protected] Stephen J. -
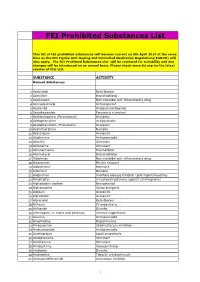
FEI Prohibited Substances List
FEI Prohibited Substances List This list of FEI prohibited substances will become current on 5th April 2010 at the same time as the FEI Equine Anti-Doping and Controlled Medication Regulations(EADCM) will also apply. The FEI Prohibted Substances List will be reviewed for suitability and any changes will be introduced on an annual basis. Please check www.fei.org for the latest version of this List. SUBSTANCE ACTIVITY Banned Substances 1 Acebutolol Beta blocker 2 Acefylline Bronchodilator 3 Acemetacin Non-steroidal anti-inflammatory drug 4 Acenocoumarol Anticoagulant 5 Acetanilid Analgesic/antipyretic 6 Acetohexamide Pancreatic stimulant 7 Acetominophen (Paracetamol) Analgesic 8 Acetophenazine Antipsychotic 9 Acetophenetidin (Phenacetin) Analgesic 10 Acetylmorphine Narcotic 11 Adinazolam Anxiolytic 12 Adiphenine Antispasmodic 13 Adrafinil Stimulant 14 Adrenaline Stimulant 15 Adrenochrome Haemostatic 16 Aformoterol Bronchodilator 17 Alclofenac Non-steroidal anti-inflammatory drug 18 Alcuronium Muscle relaxant 19 Aldosterone Hormone 20 Alfentanil Narcotic 21 Allopurinol Xanthine oxidase inhibitor (anti-hyperuricaemia) 22 Almotriptan 5-hydroxatryptamine agonist (antimigraine) 23 Alphadolone acetate Neurosteriod 24 Alphaprodine Opiod analgesic 25 Alpidem Anxiolytic 26 Alprazolam Anxiolytic 27 Alprenolol Beta blocker 28 Althesin IV anaesthetic 29 Althiazide Diuretic 30 Altrenogest (in males and geldings) Oestrus suppression 31 Alverine Antispasmodic 32 Amantadine Dopaminergic 33 Ambenonium Cholinesterase inhibition 34 Ambucetamide Antispasmodic 35 Amethocaine Local anaesthetic 36 Amfepramone Stimulant 37 Amfetaminil Stimulant 38 Amidephrine Vasoconstrictor 39 Amiloride Diuretic 40 Amineptine Tricyclic antidepressant 41 Aminoglutehthamide Aromatase inhibitor 1 FEI Prohibited Substances List This list of FEI prohibited substances will become current on 5th April 2010 at the same time as the FEI Equine Anti-Doping and Controlled Medication Regulations(EADCM) will also apply.