02 Whole.Pdf (7.606Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Evolution of Hemoglobin. by Ross Hardison
American Scientist March-April 1999 v87 i2 p126(1) Page 1 the evolution of hemoglobin. by Ross Hardison A comparative study of hemoglobin was conducted to explain how an ancestral single-function molecule gave rise to descending molecules with varied functions. Hemoglobin is the molecule in red blood cells responsible for giving blood its color and for carrying oxygen throughout the body. New functions of metallo-porphyrin rings or a kind of molecular cage embedded in proteins were developed with the appearance of atmospheric oxygen. The presence of hemoglobin in both oxygen-needing and non-oxygen needing organisms suggests the same evolutionary roots. © COPYRIGHT 1999 Sigma Xi, The Scientific Research If similar compounds are used in all of these reactions, Society then how do the different functions arise? The answer lies in the specific structure of the organic component, most Studies of a very ancient protein suggest that changes in often a protein, that houses the porphyrin ring. The gene regulation are an important part of the evolutionary configuration of each protein determines what biochemical story service the protein will perform. The appearance of atmospheric oxygen on earth between Because they have such an ancient lineage, the one and two billion years ago was a dramatic and, for the porphyrin-containing molecules provide scientists with a primitive single-celled creatures then living on earth, a rare opportunity to follow the creation of new biological potentially traumatic event. On the one hand, oxygen was compounds from existing ones. That is, how does an toxic. On the other hand, oxygen presented opportunities ancestral molecule with a single function give rise to to improve the process of metabolism, increasing the descendant molecules with varied functions? In my efficiency of life’s energy-generating systems. -

SUPPLEMENTARY DATA Data Supplement To
SUPPLEMENTARY DATA Data supplement to Perivascular adipose tissue controls insulin-stimulated perfusion, mitochondrial protein expression and glucose uptake in muscle through adipomuscular microvascular anastomoses Surname first author Turaihi Authors & Affiliations Turaihi, Alexander H MD1; Serné, Erik H, MD PhD2; Molthoff, Carla FM PhD3; Koning, Jasper J PhD4; Knol, Jaco PhD6; Niessen, Hans W MD PhD5; Goumans, Marie Jose TH PhD7; van Poelgeest, Erik M MD1; Yudkin, John S MD PhD8; Smulders, Yvo M MD PhD2; Connie R Jimenez, PhD6; van Hinsbergh, Victor WM PhD1; Eringa, Etto C PhD1 ©2020 American Diabetes Association. Published online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-1066/-/DC1 SUPPLEMENTARY DATA Western immunoblotting Skeletal muscle samples were lysed up in 1D‐sample buffer (10% glycerol, 62.5 mmol/L Tris (pH 6.8), 2% w/v LDS, 2% w/v DTT) and protein concentration was determined using Pierce 660‐nm protein assay (Thermo scientific, Waltham, MA USA 02 451; 22 660) according to the manufacturer's instructions. Heat shock protein 90 immunoblotting was performed by application of samples (5 µg protein) on 4‐15% Criterion TGX gels (Biorad, Veenendaal, the Netherlands, 5 671 084) and semi‐dry blotting onto PVDF membranes (GE Healthcare‐Fisher, RPN1416F), incubated overnight with rat monoclonal HSP90 antibody (1:1000 dilution) after blocking with 5% milk in TBS‐T (137 mM NaCl, 20 mmol/L Tris pH 7.0 and 0.1% (v/v) Tween [Sigma‐Aldrich, P7949]). After 2 hours incubation with anti-rat, horse radish peroxidase-coupled secondary antibody (Thermo Fisher 62-9520), the blot was stained using ECL‐prime (Fisher scientific, 10 308 449) and analysed on an AI‐600 imaging system (GE Healthcare, Life Sciences). -
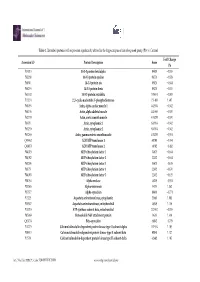
Table 1. Identified Proteins with Expression Significantly Altered in the Hippocampus of Rats of Exposed Group (Pb) Vs
Table 1. Identified proteins with expression significantly altered in the hippocampus of rats of exposed group (Pb) vs. Control. Fold Change Accession Id a Protein Description Score Pb P35213 14-3-3 protein beta/alpha 85420 −0.835 P62260 14-3-3 protein epsilon 96570 −0.878 P68511 14-3-3 protein eta 85420 −0.844 P68255 14-3-3 protein theta 85420 −0.835 P63102 14-3-3 protein zeta/delta 105051 −0.803 P13233 2',3'-cyclic-nucleotide 3'-phosphodiesterase 151400 1.405 P68035 Actin, alpha cardiac muscle 1 442584 −0.942 P68136 Actin, alpha skeletal muscle 441060 −0.970 P62738 Actin, aortic smooth muscle 438270 −0.970 P60711 Actin, cytoplasmic 1 630104 −0.942 P63259 Actin, cytoplasmic 2 630104 −0.942 P63269 Actin, gamma-enteric smooth muscle 438270 −0.951 Q05962 ADP/ATP translocase 1 60100 −0.554 Q09073 ADP/ATP translocase 2 49102 −0.482 P84079 ADP-ribosylation factor 1 34675 −0.644 P84082 ADP-ribosylation factor 2 22412 −0.644 P61206 ADP-ribosylation factor 3 34675 −0.619 P61751 ADP-ribosylation factor 4 22412 −0.670 P84083 ADP-ribosylation factor 5 22412 −0.625 P04764 Alpha-enolase 46219 −0.951 P23565 Alpha-internexin 9478 1.062 P37377 Alpha-synuclein 89619 −0.771 P13221 Aspartate aminotransferase, cytoplasmic 23661 1.083 P00507 Aspartate aminotransferase, mitochondrial 46049 1.116 P10719 ATP synthase subunit beta, mitochondrial 232442 −0.835 P85969 Beta-soluble NSF attachment protein 9638 1.419 Q63754 Beta-synuclein 66842 −0.779 P11275 Calcium/calmodulin-dependent protein kinase type II subunit alpha 181954 1.105 P08413 Calcium/calmodulin-dependent protein kinase type II subunit beta 80840 1.127 P15791 Calcium/calmodulin-dependent protein kinase type II subunit delta 62682 1.105 Int. -

The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: a Review
Journal of Clinical Medicine Review The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: A Review Felix Scholkmann 1,2,*, Tanja Restin 2, Marco Ferrari 3 and Valentina Quaresima 3 1 Biomedical Optics Research Laboratory, Department of Neonatology, University Hospital Zurich, University of Zurich, 8091 Zurich, Switzerland 2 Newborn Research Zurich, Department of Neonatology, University Hospital Zurich, University of Zurich, 8091 Zurich, Switzerland; [email protected] 3 Department of Life, Health and Environmental Sciences, University of L’Aquila, 67100 L’Aquila, Italy; [email protected] (M.F.); [email protected] (V.Q.) * Correspondence: [email protected]; Tel.: +41-4-4255-9326 Abstract: Following the outbreak of a novel coronavirus (SARS-CoV-2) associated with pneumonia in China (Corona Virus Disease 2019, COVID-19) at the end of 2019, the world is currently facing a global pandemic of infections with SARS-CoV-2 and cases of COVID-19. Since severely ill patients often show elevated methemoglobin (MetHb) and carboxyhemoglobin (COHb) concentrations in their blood as a marker of disease severity, we aimed to summarize the currently available published study results (case reports and cross-sectional studies) on MetHb and COHb concentrations in the blood of COVID-19 patients. To this end, a systematic literature research was performed. For the case of MetHb, seven publications were identified (five case reports and two cross-sectional studies), and for the case of COHb, three studies were found (two cross-sectional studies and one case report). The findings reported in the publications show that an increase in MetHb and COHb can happen in COVID-19 patients, especially in critically ill ones, and that MetHb and COHb can increase to dangerously high levels during the course of the disease in some patients. -

Fetal and Embryonic Haemoglobins P
Review Article J Med Genet: first published as 10.1136/jmg.10.1.50 on 1 March 1973. Downloaded from Journal of Medical Genetics (1973). 10, 50. Fetal and Embryonic Haemoglobins P. A. LORKIN MRC Abnormal Haemoglobin Unit, University Department of Biochemistry, Cambridge Haemoglobin has been the subject of intensive form a nearly spherical molecule with extensive research for many years and is one of the most areas of contact between unlike chains; the two thoroughly understood of all protein molecules. main types of contact are denoted alp, and alg2 The amino-acid sequences of haemoglobins from The tetramer exhibits cooperative behaviour or many species of animals have been determined haem-haem interaction. As each haem combines (tabulated by Dayhoff, 1969) and the molecular with oxygen the affinity of successive haems in- structures of horse and human haemoglobins have creases. The oxygen affinity curve of the tetramer been determined in great detail by x-ray crystallo- is sigmoidal and may be represented approximately graphy (Perutz et al, 1968a and b; Perutz 1969). A by the Hill equation:* mechanism of action of haemoglobin has been pro- = kpo2n posed (Perutz, 1970a and b and 1972). The y haemoglobins of higher organisms share a common +kpo2n tetrameric structure built up of two pairs of unlike Oxygen affinity data are usually presented in copyright. chains; the a chains containing 141 amino-acid terms of P102, the partial pressure of oxygen re- residues and the non-a chains containing generally quired to attain half saturation with oxygen, and of 145 or 146 amino acids. In man, five types of n, the exponent of the Hill equation. -

Mitochondrial Cytochrome C Oxidase As a Target Site for Daunomycin in K-562 Cells and Heart Tissue1
[CANCER RESEARCH 53. 1072-1078. March I. 1993] Mitochondrial Cytochrome c Oxidase as a Target Site for Daunomycin in K-562 Cells and Heart Tissue1 Lefkothea C. Papadopoulou and Asterios S. Tsiftsoglou2 iMhoratory of Pharmacology, Department of Pharmaceutical Sciences. Aristotle University' of Thesstiloniki. Thesstiloniki 540 (Ì6,Greece ABSTRACT cells like ADR (20); (/;) DAU interacts selectively with mitochondrial COX; these interactions appear to be specific in nature and may occur Daunomycin and other structurally related anthracyclines can cause in part via the prosthetic group of heme located in two of the several myelosuppression and cardiomyopathy. We explored the possible mecha- nism(s) by which daunomycin (DAU) interacts with target sites in neo- subunits of this enzyme; (c) DAU and ADR inhibited COX activity in plastic hemopoietic cells and heart tissue. We observed that | 'lli(. i|l) VI a dose-dependent fashion that was prevented by exogenously added interacts selectively with mitochondria! hemoproteins isolated from K-562 hemin. In light of these observations, we propose that mitochondrial cells and rat and bovine heart and forms relatively stable protein com COX may serve as a target site for DAU and presumably other plexes. Isolation, purification, and Chromatographie analysis of the mito anthracyclines on highly proliferating neoplastic cells and heart tissue. chondria! components complexed with [JH(G)]DAU revealed that one of the major components involved is cytochrome c oxidase (COX). Both DAU and ADR caused a dose-dependent inhibition of COX activity in vitro, an MATERIALS AND METHODS event prevented by exogenous hemin. The interaction of DAL with COX Chemicals and Biologicals. Hemin was purchased from Eastman Kodak appears to occur via more than one site, one of which at least appears to (Rochester. -

Linkage of Genes for Adult A-Globin and Embryonic Aulike Globin Chains (Hemoglobin H/Thalassemia/Embryonic Hemoglobin/Mice) J
Proc. Natl. Acad. Sci. USA Vol. 77, No. 2, pp. 1087-1090, February 1980 Genetics Linkage of genes for adult a-globin and embryonic aulike globin chains (hemoglobin H/thalassemia/embryonic hemoglobin/mice) J. BARRY WHITNEY III* AND ELIZABETH S. RUSSELL The Jackson Laboratory, Bar Harbor, Maine 04609 contributed by Elizabeth S. Russell, November 19, 1979 ABSTRACT In a-thalassemia, the genetic locus for the a (Hbaa) female mouse and a triethylene-melamine-treated male chains of adult hemoglobin is not expressed. We have examined that carried a different, doublet, Hba haplotype (5). In this the hemoglobins of a number of individual mouse embryos affected a-thalassemic offspring of the treated male, only the heterozygous for a particular a-thalassemia (Hba th-J) and find n'o.'ecrease in the proportion of hemoglobins containing the Hbaa haplotype inherited from the C57BL/6J mother was a chain as compared to the hemoglobin containing the a-like expressed. The hemoglobins of this mouse were found by embryonic globin chain. This result suggests that the locus for electrophoresis after cystamine treatment (6) to be unusual in this embryonic a-like chain is inactivated or deleted in these that they contained a lower than normal proportion of the he- embryos as well. Because a single mutational event inactivated moglobin containing the diffuse-major:/ chain, a condition also adult and embryonic loci, we conclude that they are probably reported for the x-ray-induced mouse a-thalassemia discovered closely linked to one another on the same chromosome. We also present evidence that an unusual hemoglobin in the blood of at the Oak Ridge National Laboratory (7). -

Diabetes-Induced Mitochondrial Dysfunction in the Retina
Diabetes-Induced Mitochondrial Dysfunction in the Retina Renu A. Kowluru and Saiyeda Noor Abbas 4–9 PURPOSE. Oxidative stress is increased in the retina in diabetes, tase are downregulated. We have reported that the long- and antioxidants inhibit activation of caspase-3 and the devel- term administration of antioxidants inhibits the development opment of retinopathy. The purpose of this study was to of retinopathy in diabetic rats and in galactose-fed rats (another investigate the effect of diabetes on the release of cytochrome model of diabetic retinopathy),3 suggesting an important role c from mitochondria and translocation of Bax into mitochon- for oxidative stress in the development of retinopathy in dia- dria in the rat retina and in the isolated retinal capillary cells. betes. Oxidative stress is involved directly in the upregulation ETHODS of vascular endothelial growth factor in the retina during early M . Mitochondria and cytosol fractions were prepared 10 from retina of rats with streptozotocin-induced diabetes and diabetes. Recent studies from our laboratory have shown that from the isolated retinal endothelial cells and pericytes incu- oxidative stress plays an important role, not only in the devel- opment of retinopathy in diabetes, but also in the resistance of bated in 5 or 20 mM glucose medium for up to 10 days in the 11 presence of superoxide dismutase (SOD) or a synthetic mi- retinopathy to arrest after good glycemic control is initiated. metic of SOD (MnTBAP). The release of cytochrome c into the Capillary cells and neurons are lost in the retina before other histopathology is detectable, and apoptosis has been cytosol and translocation of the proapoptotic protein Bax into 12–15 the mitochondria were determined by the Western blot tech- implicated as one of the mechanism(s). -

Iron Deficiency in the Rat: Effects on Neutrophil Activation and Metabolism
THEOPHYLLINE AND BRAIN 549 A dietary protein deficiency can affect the susceptibility to the metabolism in children with protein-calorie malnutrition. Am. J. Clin. Nutr., 28: 977 (1975). toxicity of drugs or other agents such as pesticides and herbicides 8. Nakamoto. T. and Miller. S. A.: Effect of vrotein-energy malnutrition on the (2). At the present time, there are no such studies relative to growth df mandible aid long bone in newborn miie and female rats. J. theophylline, although the usage of theophylline is now quite Nutr., 107: 983 (1977). common in the neonatal intensive care environment. Many 9. Nebron, R. M., Resnick, M. D., and Halstmm, W. J.: Developmental outcome of premature infants treated with theophylline. Dev. Pharmacol. Ther., 1: infants therein who are now receiving theophylline therapeuti- 274 ( 1980). cally are being dosed on a body weight basis without considera- 10. Newberne, P. M., Gross, R. L., and Roe, D. A,: Dmg, toxin, nutrient interac- tion of the nutritional status. Our data suggest that, in the animal tions. World Rev. Nutr. Diet., 29: 130 (1978). model, the administration of theophylline in the presence of a 1 I. Pastorova, B., Sova, O., and Burda, J.: Incorporation of I4C-thymidine into liver and brain DNA of protein-deficient rats. Physiol. Bohemoslov., 27: 69 compromised nutritional status may have effects not now appar- (1978). ent. We add our concern to that expressed by others (16) that 12. Prasad, A. S., Dumouchell, E., Kovich, D., and Oberleas, D.: A simple methylxanthine administration may have previously unsus- fluorometric method for the determination of RNA and DNA in tissue. -

Hemoglobin, Myoglobin and Neuroglobin in Endogenous Thiosulfate Production Processes
International Journal of Molecular Sciences Review The Role of Hemoproteins: Hemoglobin, Myoglobin and Neuroglobin in Endogenous Thiosulfate Production Processes Anna Bilska-Wilkosz *, Małgorzata Iciek, Magdalena Górny and Danuta Kowalczyk-Pachel Chair of Medical Biochemistry, Jagiellonian University Collegium Medicum ,7 Kopernika Street, Kraków 31-034, Poland; [email protected] (M.I.); [email protected] (M.G.); [email protected] (D.K.-P.) * Correspondence: [email protected]; Tel.: +48-12-4227-400; Fax: +48-12-4223-272 Received: 5 May 2017; Accepted: 16 June 2017; Published: 20 June 2017 Abstract: Thiosulfate formation and biodegradation processes link aerobic and anaerobic metabolism of cysteine. In these reactions, sulfite formed from thiosulfate is oxidized to sulfate while hydrogen sulfide is transformed into thiosulfate. These processes occurring mostly in mitochondria are described as a canonical hydrogen sulfide oxidation pathway. In this review, we discuss the current state of knowledge on the interactions between hydrogen sulfide and hemoglobin, myoglobin and neuroglobin and postulate that thiosulfate is a metabolically important product of this processes. Hydrogen sulfide oxidation by ferric hemoglobin, myoglobin and neuroglobin has been defined as a non-canonical hydrogen sulfide oxidation pathway. Until recently, it appeared that the goal of thiosulfate production was to delay irreversible oxidation of hydrogen sulfide to sulfate excreted in urine; while thiosulfate itself was only an intermediate, transient metabolite on the hydrogen sulfide oxidation pathway. In the light of data presented in this paper, it seems that thiosulfate is a molecule that plays a prominent role in the human body. Thus, we hope that all these findings will encourage further studies on the role of hemoproteins in the formation of this undoubtedly fascinating molecule and on the mechanisms responsible for its biological activity in the human body. -

Adult, Embryonic and Fetal Hemoglobin Are Expressed in Human Glioblastoma Cells
514 INTERNATIONAL JOURNAL OF ONCOLOGY 44: 514-520, 2014 Adult, embryonic and fetal hemoglobin are expressed in human glioblastoma cells MARWAN EMARA1,2, A. ROBERT TURNER1 and JOAN ALLALUNIS-TURNER1 1Department of Oncology, University of Alberta and Alberta Health Services, Cross Cancer Institute, Edmonton, AB T6G 1Z2, Canada; 2Center for Aging and Associated Diseases, Zewail City of Science and Technology, Cairo, Egypt Received September 7, 2013; Accepted October 7, 2013 DOI: 10.3892/ijo.2013.2186 Abstract. Hemoglobin is a hemoprotein, produced mainly in Introduction erythrocytes circulating in the blood. However, non-erythroid hemoglobins have been previously reported in other cell Globins are hemo-containing proteins, have the ability to types including human and rodent neurons of embryonic bind gaseous ligands [oxygen (O2), nitric oxide (NO) and and adult brain, but not astrocytes and oligodendrocytes. carbon monoxide (CO)] reversibly. They have been described Human glioblastoma multiforme (GBM) is the most aggres- in prokaryotes, fungi, plants and animals with an enormous sive tumor among gliomas. However, despite extensive basic diversity of structure and function (1). To date, hemoglobin, and clinical research studies on GBM cells, little is known myoglobin, neuroglobin (Ngb) and cytoglobin (Cygb) repre- about glial defence mechanisms that allow these cells to sent the vertebrate globin family with distinct function and survive and resist various types of treatment. We have tissue distributions (2). During ontogeny, developing erythro- shown previously that the newest members of vertebrate blasts sequentially express embryonic {[Gower 1 (ζ2ε2), globin family, neuroglobin (Ngb) and cytoglobin (Cygb), are Gower 2 (α2ε2), and Portland 1 (ζ2γ2)] to fetal [Hb F(α2γ2)] expressed in human GBM cells. -

The Effects of Temperature on Hemoglobin in Capitella Teleta
THE EFFECTS OF TEMPERATURE ON HEMOGLOBIN IN CAPITELLA TELETA by Alexander M. Barclay A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Master of Science in Marine Studies Summer 2013 c 2013 Alexander M. Barclay All Rights Reserved THE EFFECTS OF TEMPERATURE ON HEMOGLOBIN IN CAPITELLA TELETA by Alexander M. Barclay Approved: Adam G. Marsh, Ph.D. Professor in charge of thesis on behalf of the Advisory Committee Approved: Mark A. Moline, Ph.D. Director of the School of Marine Science and Policy Approved: Nancy M. Targett, Ph.D. Dean of the College of Earth, Ocean, and Environment Approved: James G. Richards, Ph.D. Vice Provost for Graduate and Professional Education ACKNOWLEDGMENTS I extend my sincere gratitude to the individuals that either contributed to the execution of my thesis project or to my experience here at the University of Delaware. I would like to give special thanks to my adviser, Adam Marsh, who afforded me an opportunity that exceeded all of my prior expectations. Adam will say that I worked very independently and did not require much guidance, but he served as an inspiration and a role model for me during my studies. Adam sincerely cares for each of his students and takes the time and thought to tailor his research program to fit each person's interests. The reason that I initially chose to work in Adam's lab was that he expressed a genuine excitement for science and for his lifes work. His enthusiasm resonated with me and helped me to find my own exciting path in science.