Blend of Polypropylene/Poly(Lactic Acid) for Medical Packaging Application: Physicochemical, Thermal, Mechanical, and Barrier Properties
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Film Performance of Poly(Lactic Acid) Blends for Packaging Applications
RESEARCH ARTICLE Film Performance of Poly(lactic acid) Blends for Packaging Applications PREFACE API 2015 Carlos Diaz Hsun Yi (Sarah) Pao Rochester Institute of Technology Rochester Institute of Technology [email protected] [email protected] Sungyoung Kim Rochester Institute of Technology [email protected] ABSTRACT Poly(lactic acid) (PLA), a biodegradable thermoplastic derived from renewable resources, stands out as a substitute to petroleum-based plastics. PLA based films for food packaging has been an area of both commercial and research interest within the context of sustainability. In spite of its high strength, packaging applications have been limited because PLA is more brittle than traditional oil-based plastics. Because of this, films display low tear and impact resistance and produce a loud crackling sound when manipulated. Although many studies address the toughening of PLA in the bulk, little attention has been placed on the film performance. The present study is aimed at providing a survey of binary PLA based blends with other biodegradable and non-biodegradable plastics. Acrylic impact modifier (AIM, 5 wt. %), ethylene vinyl acetate (EVA, 20 wt.%), polyhydroxyalkanoate (PHA, 10 wt.%), polycaprolactone (PCL, 30 wt.%), polybutylene succinate (PBS, 20 wt.%) and polypropylene carbonate (PPC, 30 wt.%) were each blended with PLA through single-screw extrusion and converted into films via the blown-film process. Tear and impact resistance, heat seal strength, and noise level were measured. EVA, PHA, PCL, and PBS improved the tear resistance with EVA having the highest effect (>2x). Similarly AIM, EVA and PPC improved the resistance of the film to impact-puncture penetration. Heat seal strength was significantly improved by the PHA and moderately increased by AIM (2x) and EVA. -

Modified PPE-PS Americas
Noryl* resin Modifi ed PPE-PS Americas 2 SABIC Innovative Plastics 1. Introduction Noryl* resin is based on a modified PPO technology developed by SABIC Innovative Plastics. Noryl resin is an extremely versatile material - a miscible blend of PPO resin and polystyrene - and the basic properties may be modified to achieve a variety of characteristics. Contents 1. Introduction 4 2. Applications 6 3. Properties and design 10 3.1 General properties 10 3.2 Mechanical properties 10 3.3 Electrical properties 13 3.4 Flammability 13 3.5 Environmental resistance 14 3.6 Processability 14 3.7 Mold shrinkage 15 4. Processing 16 4.1 Pre-drying 16 4.2 Equipment 16 4.3 Processing conditions 17 4.4 Purging of the barrel 17 4.5 Recycling 17 5. Secondary operations 18 5.1 Welding 18 5.2 Adhesives 19 5.3 Mechanical assembly 20 5.4 Painting 20 5.5 Metalization 21 5.6 Laser marking 22 5.7 Foaming 22 SABIC Innovative Plastics 3 1. Introduction Noryl* modifi ed PPO resin PPE-PS Characteristics Typical characteristics include excellent dimensional stability, low mold shrinkage, low water absorption and very low creep behavior at elevated temperatures. These properties combined with an outstanding hydrolytic stability in hot and cold water, make Noryl resin an excellent potential candidate for fluid engineering, environmental and potable water applications. An outstanding feature of Noryl resin is its retention of tensile and flexural strength, even at elevated temperatures. The gradual reduction in modulus as temperature is increased, is a key advantage of this material. As a result, parts made or molded from Noryl resin may be used with predictable performance over a wide temperature range. -

Poly(Phenylene Ether) Based Amphiphilic Block Copolymers
polymers Review ReviewPoly(phenylene ether) Based Amphiphilic Poly(phenyleneBlock Copolymers ether) Based Amphiphilic Block Copolymers Edward N. Peters EdwardSABIC, Selkirk, N. Peters NY 12158, USA; [email protected]; Tel.: +1-518-475-5458 SABIC, Selkirk, NY 12158, USA; [email protected]; Tel.: +1-518-475-5458 Received: 14 August 2017; Accepted: 5 September 2017; Published: 8 September 2017 Received: 14 August 2017; Accepted: 5 September 2017; Published: 8 September 2017 Abstract: Polyphenylene ether (PPE) telechelic macromonomers are unique hydrophobic polyols Abstract: Polyphenylene ether (PPE) telechelic macromonomers are unique hydrophobic polyols which have been used to prepare amphiphilic block copolymers. Various polymer compositions which have been used to prepare amphiphilic block copolymers. Various polymer compositions have have been synthesized with hydrophilic blocks. Their macromolecular nature affords a range of been synthesized with hydrophilic blocks. Their macromolecular nature affords a range of structures structures including random, alternating, and di- and triblock copolymers. New macromolecular including random, alternating, and di- and triblock copolymers. New macromolecular architectures architectures can offer tailored property profiles for optimum performance. Besides reducing can offer tailored property profiles for optimum performance. Besides reducing moisture uptake and moisture uptake and making the polymer surface more hydrophobic, the PPE hydrophobic segment making the polymer surface more hydrophobic, the PPE hydrophobic segment has good compatibility has good compatibility with polystyrene (polystyrene-philic). In general, the PPE contributes to the with polystyrene (polystyrene-philic). In general, the PPE contributes to the toughness, strength, toughness, strength, and thermal performance. Hydrophilic segments go beyond their affinity for and thermal performance. Hydrophilic segments go beyond their affinity for water. -

Mechanical Behaviour of Polylactic Acid Foam As Insulation Under Increasing Temperature
Environmental and Climate Technologies 2019, vol. 23, no. 3, pp. 202–210 doi: 10.2478/rtuect-2019-0090 https://content.sciendo.com Mechanical Behaviour of Polylactic Acid Foam as Insulation Under Increasing Temperature Lucia DOYLE1*, Ingo WEIDLICH2 1,2 HafenCity University, Uberseeallee 16, Hamburg, 20457, Germany Abstract – Measures to increase the share of renewables in heat generation, combined with increased energy efficiency provide a direct emissions reduction on the heating sector. Energy efficiency measures, as well as the role-out of sustainable heating technologies such as district heating networks have one key actor: insulation. However, state of the art insulating materials such as polyurethane or polystyrene have severe environmental drawbacks incompatible with today’s transition to the circular economy, and are the Achilles’ heel of the sector in terms of sustainability. Biobased and biodegradable polylactic acid (PLA) foam could be a promising replacement for fossil-based polymeric insulating foams. This study provides data on the mechanical behaviour of expanded PLA foam under different temperatures, which will help to assess its potential use as insulation where the foam is subject to heat. Keywords – Circular economy; insulating foam; Polylactic Acid (PLA); thermal behaviour Nomenclature σ10 Compressive Stress at 10 % strain MPa E Young Modulus MPa T Temperature °C Tg Glass transition temperature °C a Sample width mm b Sample depth mm h Sample height mm 1. INTRODUCTION Energy efficiency measures combined with sustainable renewable sources of heat would significantly reduce global CO2 emissions and contribute to the achievement of goals such as the EU 2020 climate and energy package. The 4th generation District Heating [1] aims in this direction: by reducing the network´s temperature, up to now non-used waste and renewable heat sources can be introduced in the system, significantly contributing to the decarbonisation of the sector, as well as contributing to higher air quality in urban areas. -
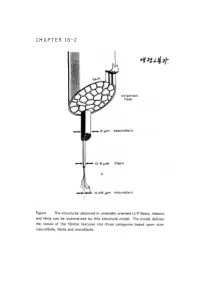
Chapter 16-2
CHAPTER 16-2 ● Polymer Blends (reference) 1. Polymer-polymer Miscibility By Olabisi, Robeson, and Shaw Academic Press(1979) 2. Poplymer Blends, by D.R Paul, Ed, Academic Press (1978). 3. specific Interactions and the Miscibility of Polymer Blends, by Coleman, Graf, and Painter(1990) 4. Polymer Alloys and Blends, Thermodynamics and Rheology, by L.A. Utracki,(1990). ● Blend Preparation 방법: (a) Melt blending- screw extruder를 사용하여 blend하고자 하는 고분자를 용융점 이상에서 extrusion하여 mixing함. (b) Solution blending- mixing하고자 하는 고분자의 cosolvent를 사용하여 solution으로 만든 다음 cast하여 필름(film) 상태 로 blend를 제조함. (ex) polymer membrane(분리막)제조시. 그러나, amorphous polymer와 crystalline polymer를 blend했을때 는 film의 투명성으로부터 사용성을 판별하기는 어렵다. (2)DSC(differential scanning calorimetry): 시차 주사 열분석기를 이용하여 블렌드의 Tg(유리전이 온도)를 측 정함으로서 상용성을 관찰함-가장 많이 쓰이는 방법중 하나임. 즉 (i) a single phase exhibits one Tg. (ex) A polymer : Polycarbonate, Tg=150°C (423 K) B polymer : Polycaprolactane, Tg=-52°C (221 K) Blend (A+B)/50:50인 경우 w w 1 = A + B Tg Blend Tg A Tg B 1 = 0.5 + 0.5 (TgBlend=17.3°C (290.3 K) Tg Blend 423 221 (2) microscopy method (Scanning Electron microscopy). (3) FT-IR (Fourier Transform IR) (4) Ternary solution method (polymer 1- polymer 2 - solvent) - Compatible components from a single, transparent phase in mutual solution, while incompatible polymers exhibit phase seperation if the solution is not extremely dilute. - Equilibrium is relatively easily achived in dilutions and - Blends of immiscible (or partially miscible) materials can be useful so long as no significant desegration occurs while the mixture is being mixed. -

Melt Free-Radical Grafting of Maleic Anhydride Onto Biodegradable Poly(Lactic Acid) by Using Styrene As a Comonomer
Polymers 2014, 6, 1528-1543; doi:10.3390/polym6051528 OPEN ACCESS polymers ISSN 2073-4360 www.mdpi.com/journal/polymers Article Melt Free-Radical Grafting of Maleic Anhydride onto Biodegradable Poly(lactic acid) by Using Styrene as a Comonomer Piming Ma, Long Jiang, Tao Ye, Weifu Dong and Mingqing Chen * The Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 1800 Lihu Road, Wuxi 214122, China; E-Mails: [email protected] (P.M.); [email protected] (L.J.); [email protected] (T.Y.); [email protected] (W.D.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +86-510-8591-7019; Fax: +86-510-8591-7763. Received: 15 April 2014; in revised form: 6 May 2014 / Accepted: 6 May 2014 / Published: 21 May 2014 Abstract: Maleic anhydride (MA) was grafted onto poly(lactic acid) (PLA) in the presence of styrene (St) by using a free-radical grafting methodology. The grafting degree (Dg) of MA was increased from 0.65 wt % to 1.1 wt % with the St/MA ratio up to 2/1, where the grafting efficiency (Eg) of MA was 27%. However, both Dg and Eg were decreased with further increasing of the St/MA ratio to 4/1. The Dg of MA increased with MA concentration and showed a maximum at 180 °C in the temperature range of 165 °C–190 °C. The grafting mechanisms of MA in the presence of St are analyzed based on titration, thermogravimetric analysis and infrared results, i.e., MA is grafted onto PLA chains via single monomers and a charge-transfer-complex (CTC) at St/MA ratios of ≤ 1/1, while dominantly via St-co-MA oligomers at St/MA ratios of around 2/1. -

Polylactic Acid
Inspirational chemistry 71 Polylactic acid Index 3.1.10 2 sheets Index 3.1.11 3 sheets This experiment is aimed only at more able and more sensible students as an ! introduction to other studies on polylactic acid. It is not recommended for students who would find it difficult to be calm and concentrated while heating a liquid to a high temperature for at least 10 minutes. The student sheet Using polylactic acid covers some of the environmental issues surrounding the industrial production of this plastic and its disposal. Background information Lactic acid (2-hydroxypropanoic acid) can be obtained by fermenting glucose or maltose or can be extracted from milk. It is used as the starting point for the production of polylactic acid, also known as poly (2-hydroxypropanoic acid), which can also be made from petrochemicals. Polylactic acid is a condensation polymer – a molecule of water is produced for every link made in the chain. This is not specified in the students’ notes, but you might wish to draw their attention to it. When the water is removed, an oligomer made of 10–30 lactic acid units is formed. An oligomer is essentially a short length of polymer. The production of true polylactic acid involves catalytic depolymerisation of the oligomer to a lactide intermediate followed by polymerisation of the lactide to high molecular weight polylactic acid. In the experiment described here only the oligomer is made. The product therefore has different properties from the polylactic acid used in packaging. It may be useful to discuss with students how the different properties are related to the chain length of the polymer molecules. -

Determination of Polymer Blend Composition, TS-22
Thermal Analysis & Rheology THERMAL SOLUTIONS DETERMINATION OF POLYMER BLEND COMPOSITION PROBLEM SOLUTION The blending of two or more polymers is becoming a Conventional DSC is a technique which measures the total common method for developing new materials for demand- heat flow into and out of a material as a function of ing applications such as impact-resistant parts and temperature and/or time. Hence, conventional DSC packaging films. Since the ultimate properties of blends can measures the sum of all thermal events occurring in the TM be significantly affected by what polymers are present, as material. Modulated DSC , on the other hand, is a new well as by small changes in the blend composition, technique which subjects a material to a linear heating suppliers of these materials are interested in rapid tests method which has a superimposed sinusoidal temperature which provide verification that the correct polymers and oscillation (modulation) resulting in a cyclic heating profile. amount of each polymer are present in the blend. Differen- tial scanning calorimetry (DSC) has proven to be an Deconvolution of the resultant heat flow profile during this effective technique for characterizing blends such as cyclic heating provides not only the "total" heat flow polyethylene/polypropylene where the crystalline melting obtained from conventional DSC, but also separates that endotherms or other transitions (e.g., glass transition) "total" heat flow into its heat capacity-related (reversing) associated with the polymer components are sufficiently and kinetic (nonreversing) components. It is this separa- TM separated to allow identification and/or quantitation. tion aspect which allows MDSC to more completely However, many blends do not exhibit this convenient evaluate polymer blend compositions. -

Spherical Polybutylene Terephthalate (PBT)—Polycarbonate (PC) Blend Particles by Mechanical Alloying and Thermal Rounding
polymers Article Spherical Polybutylene Terephthalate (PBT)—Polycarbonate (PC) Blend Particles by Mechanical Alloying and Thermal Rounding Maximilian A. Dechet 1,2,3,†, Juan S. Gómez Bonilla 1,2,3,†, Lydia Lanzl 3,4, Dietmar Drummer 3,4, Andreas Bück 1,2,3, Jochen Schmidt 1,2,3 and Wolfgang Peukert 1,2,3,* 1 Institute of Particle Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Cauerstraße 4, D-91058 Erlangen, Germany; [email protected] (M.A.D.); [email protected] (J.S.G.B.); [email protected] (A.B.); [email protected] (J.S.) 2 Interdisciplinary Center for Functional Particle Systems, Friedrich-Alexander-Universität Erlangen-Nürnberg, Haberstraße 9a, D-91058 Erlangen, Germany 3 Collaborative Research Center 814—Additive Manufacturing, Am Weichselgarten 9, D-91058 Erlangen, Germany; [email protected] (L.L.); [email protected] (D.D.) 4 Institute of Polymer Technology, Friedrich-Alexander-Universität Erlangen-Nürnberg, Am Weichselgarten 9, D-91058 Erlangen, Germany * Correspondence: [email protected]; Tel.: +49-9131-85-29400 † The authors contributed equally. Received: 22 November 2018; Accepted: 7 December 2018; Published: 11 December 2018 Abstract: In this study, the feasibility of co-grinding and the subsequent thermal rounding to produce spherical polymer blend particles for selective laser sintering (SLS) is demonstrated for polybutylene terephthalate (PBT) and polycarbonate (PC). The polymers are jointly comminuted in a planetary ball mill, and the obtained product particles are rounded in a heated downer reactor. The size distribution of PBT–PC composite particles is characterized with laser diffraction particle sizing, while the shape and morphology are investigated via scanning electron microscopy (SEM). -

TA No.81 Thermal Analysis of Polylactic Acid -Crystallinity and Heat Resistance- 2007.9
TA no.81 Thermal analysis of polylactic acid -Crystallinity and heat resistance- 2007.9 1. Introduction 10mg samples were heated from 20 °C to 200 °C Recent views on waste disposal and environmental at 10 °C /min. in a nitrogen atmosphere. For the conservation have increased the focus on biode- TG measurements, 10-mg samples were heated from gradable plastic, which can be produced from re- room temperature to 400 °C at rates of 10, 5, 2 and newable raw materials and decomposes in the natural 1 °C / min. in a nitrogen atmosphere. environment. Polylactic acid (PLA) is a biodegrad- 3. Results able plastic derived from plants that is widely used in packing materials, fibers and medical materials. Figure 1 shows the DSC results when the samples Crystallinity is an important consideration for the were melted and then cooled at 0.1 °C / min. strength, impact resistance, and transparency re- Figure 2 shows the DSC results when the samples quirements of these materials and influences biode- were quenched rapidly. gradability. Furthermore, lactic acid, the PLA As seen in Figure 1, glass transition (Tg) occurred monomer, has asymmetrical carbon atoms and thus around 60 °C for all samples. Melting occurred with has optical isomers. The isomeric ratio and molecu- endothermic peaks at 150 °C and 170 °C for lar weight of polymers influence crystallinity and heat Sample b and Sample c/ c', respectively. These resistance, so their roles must be considered during results show that the higher the L ratio, the easier it the formation process. is for crystallization to occur. -

FUNCTIONAL PLA BASED SYSTEMS a Dissertation Presented to the Graduate Faculty of the University of Akron in Partial Fulfillment
FUNCTIONAL PLA BASED SYSTEMS A Dissertation Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Colin Wright December, 2015 FUNCTIONAL PLA BASED SYSTEMS Colin Wright Dissertation Approved: Accepted: ________________________________ ____________________________________ Advisor Department Chair Dr. Coleen Pugh Dr. Coleen Pugh ________________________________ ____________________________________ Committee Chair Dean of the College Dr. Robert Weiss Dr. Eric Amis ________________________________ ____________________________________ Committee Member Dean of the Graduate School Dr. Mathew Becker Dr. Chand Midha ________________________________ ____________________________________ Committee Member Date Dr. William Landis ________________________________ Committee Member Dr. Yang Yun ii ABSTRACT Poly(lactic acid) (PLA), is used in a wide variety of applications. It is a well studied polymer and offers many advantages, such as being derived from renewable resources, being biodegradable, FDA approved for biomedical applications, and commercially available. The main synthetic drawback is that the only sites for post-polymerization functionalization are at the two end groups. By incorporating 3-hydroxy-2- bromopropionic acid as a co-monomer with lactic acid, a site for post-polymerization functionalization can be added. Since the halogen is alpha to a carbonyl, it is activated toward nucleophlic substitution, radical formation, and enolate chemistry. The spacing on the backbone of our polymer allows for additional functionalization including rearrangement, electrophilic aromatic substitution, and cationic ring-opening polymerization. iii DEDICATION I would like to dedicate this dissertation to my parents for encouraging me to attend graduate school. iv ACKNOWLEDGMENTS I would like to thank my mother and father for their unfailing support of me during my time in academia. -

Designing Polymer Blends Using Neural Networks, Genetic
Designing Polymer Blends Using Neural Networks, Genetic Algorithms, and Markov Chains N. K. Roy1,2, W. D. Potter1, D. P. Landau2 1Department of Computer Science University of Georgia, Athens, GA 30602 2Center for Simulational Physics University of Georgia, Athens, GA 30602 ABSTRACT In this paper we present a new technique to simulate polymer blends that overcomes the shortcomings in polymer system modeling. This method has an inherent advantage in that the vast existing information on polymer systems forms a critical part in the design process. The stages in the design begin with selecting potential candidates for blending using Neural Networks. Generally the parent polymers of the blend need to have certain properties and if the blend is miscible then it will reflect the properties of the parents. Once this step is finished the entire problem is encoded into a genetic algorithm using various models as fitness functions. We select the lattice fluid model of Sanchez and Lacombe1, which allows for a compressible lattice. After reaching a steady-state with the genetic algorithm we transform the now stochastic problem that satisfies detailed balance and the condition of ergodicity to a Markov Chain of states. This is done by first creating a transition matrix, and then using it on the incidence vector obtained from the final populations of the genetic algorithm. The resulting vector is converted back into a 1 population of individuals that can be searched to find the individuals with the best fitness values. A high degree of convergence not seen using the genetic algorithm alone is obtained. We check this method with known systems that are miscible and then use it to predict miscibility on several unknown systems.