Meltwater‐Enhanced Nutrient Export from Greenland's Glacial Fjords: A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geologic Maps of the Eastern Alaska Range, Alaska, (44 Quadrangles, 1:63360 Scale)
Report of Investigations 2015-6 GEOLOGIC MAPS OF THE EASTERN ALASKA RANGE, ALASKA, (44 quadrangles, 1:63,360 scale) descriptions and interpretations of map units by Warren J. Nokleberg, John N. Aleinikoff, Gerard C. Bond, Oscar J. Ferrians, Jr., Paige L. Herzon, Ian M. Lange, Ronny T. Miyaoka, Donald H. Richter, Carl E. Schwab, Steven R. Silva, Thomas E. Smith, and Richard E. Zehner Southeastern Tanana Basin Southern Yukon–Tanana Upland and Terrane Delta River Granite Jarvis Mountain Aurora Peak Creek Terrane Hines Creek Fault Black Rapids Glacier Jarvis Creek Glacier Subterrane - Southern Yukon–Tanana Terrane Windy Terrane Denali Denali Fault Fault East Susitna Canwell Batholith Glacier Maclaren Glacier McCallum Creek- Metamorhic Belt Meteor Peak Slate Creek Thrust Broxson Gulch Fault Thrust Rainbow Mountain Slana River Subterrane, Wrangellia Terrane Phelan Delta Creek River Highway Slana River Subterrane, Wrangellia Terrane Published by STATE OF ALASKA DEPARTMENT OF NATURAL RESOURCES DIVISION OF GEOLOGICAL & GEOPHYSICAL SURVEYS 2015 GEOLOGIC MAPS OF THE EASTERN ALASKA RANGE, ALASKA, (44 quadrangles, 1:63,360 scale) descriptions and interpretations of map units Warren J. Nokleberg, John N. Aleinikoff, Gerard C. Bond, Oscar J. Ferrians, Jr., Paige L. Herzon, Ian M. Lange, Ronny T. Miyaoka, Donald H. Richter, Carl E. Schwab, Steven R. Silva, Thomas E. Smith, and Richard E. Zehner COVER: View toward the north across the eastern Alaska Range and into the southern Yukon–Tanana Upland highlighting geologic, structural, and geomorphic features. View is across the central Mount Hayes Quadrangle and is centered on the Delta River, Richardson Highway, and Trans-Alaska Pipeline System (TAPS). Major geologic features, from south to north, are: (1) the Slana River Subterrane, Wrangellia Terrane; (2) the Maclaren Terrane containing the Maclaren Glacier Metamorphic Belt to the south and the East Susitna Batholith to the north; (3) the Windy Terrane; (4) the Aurora Peak Terrane; and (5) the Jarvis Creek Glacier Subterrane of the Yukon–Tanana Terrane. -
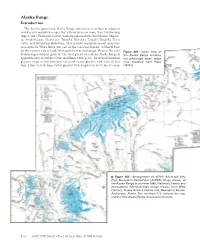
Alaska Range
Alaska Range Introduction The heavily glacierized Alaska Range consists of a number of adjacent and discrete mountain ranges that extend in an arc more than 750 km long (figs. 1, 381). From east to west, named ranges include the Nutzotin, Mentas- ta, Amphitheater, Clearwater, Tokosha, Kichatna, Teocalli, Tordrillo, Terra Cotta, and Revelation Mountains. This arcuate mountain massif spans the area from the White River, just east of the Canadian Border, to Merrill Pass on the western side of Cook Inlet southwest of Anchorage. Many of the indi- Figure 381.—Index map of vidual ranges support glaciers. The total glacier area of the Alaska Range is the Alaska Range showing 2 approximately 13,900 km (Post and Meier, 1980, p. 45). Its several thousand the glacierized areas. Index glaciers range in size from tiny unnamed cirque glaciers with areas of less map modified from Field than 1 km2 to very large valley glaciers with lengths up to 76 km (Denton (1975a). Figure 382.—Enlargement of NOAA Advanced Very High Resolution Radiometer (AVHRR) image mosaic of the Alaska Range in summer 1995. National Oceanic and Atmospheric Administration image mosaic from Mike Fleming, Alaska Science Center, U.S. Geological Survey, Anchorage, Alaska. The numbers 1–5 indicate the seg- ments of the Alaska Range discussed in the text. K406 SATELLITE IMAGE ATLAS OF GLACIERS OF THE WORLD and Field, 1975a, p. 575) and areas of greater than 500 km2. Alaska Range glaciers extend in elevation from above 6,000 m, near the summit of Mount McKinley, to slightly more than 100 m above sea level at Capps and Triumvi- rate Glaciers in the southwestern part of the range. -

Comparison of Four Calving Laws to Model Greenland Outlet Glaciers Youngmin Choi1, Mathieu Morlighem1, Michael Wood1, and Johannes H
The Cryosphere Discuss., https://doi.org/10.5194/tc-2018-132 Manuscript under review for journal The Cryosphere Discussion started: 23 July 2018 c Author(s) 2018. CC BY 4.0 License. Comparison of four calving laws to model Greenland outlet glaciers Youngmin Choi1, Mathieu Morlighem1, Michael Wood1, and Johannes H. Bondzio1 1University of California, Irvine, Department of Earth System Science, 3218 Croul Hall, Irvine, CA 92697-3100, USA Correspondence to: Youngmin Choi ([email protected]) Abstract. Calving is an important mechanism that controls the dynamics of marine terminating glaciers of Greenland. Ice- berg calving at the terminus affects the entire stress regime of outlet glaciers, which may lead to further retreat and ice flow acceleration. It is therefore critical to accurately parameterize calving in ice sheet models in order to improve the projections of ice sheet change over the coming decades and reduce the uncertainty in their contribution to sea level rise. Several calving 5 laws have been proposed, but most of them have been applied only to a specific region and have not been tested on other glaciers, while some others have only been implemented in one-dimensional flowline or vertical flowband models. Here, we test and compare several calving laws recently proposed in the literature using the Ice Sheet System Model (ISSM). We test these calving laws on nine tidewater glaciers of Greenland. We compare the modeled ice front evolution to the observed retreat from Landsat data collected over the past 10 years, and assess which calving law has better predictive abilities for each glacier. 10 Overall, the von Mises tensile stress calving law is more satisfactory than other laws for simulating observed ice front retreat, but new parameterizations that capture better the different modes of calving should be developed. -

Harvard Mountaineering 6
, "HARVARD MOUNTAINEERING • Number 6 APRIL · 1943 THE THE HARVARP MOUNTAINEERING CLUB CAMBRIDGE, MASS. HARVARD MOUNTAINEERING NUMBER 6 APRIL, 1943 THE HARVARD MOUNTAINEERING CLUB CAMBRIDGE, MASS. Contents CLUB OFFICERS 4 FOREWORD 5 MT. BERTHA, FAIRWEATHER RANGE, 1940 . 7 THE H. M. C. 1941 EXPEDITION TO PERU 15 MT. HAYES 18 THE FIRST ASCENT OF MT. BAGLEY 23 MT. \VOOD AND MT. WALSH, ST. ELIAS RANGE 27 RETURN TO GLACIER BAY 30 MT. McKINLEY IN WAR TIME . 33 CASCADES-DRY AND WET 36 HARVARD AT GLACIER CIRCLE 4] OTHER· CLIMBS AND EXPEDITIONS 47 SPUR CABIN . 51 DIGEST OF LOCAL ROCK AND ICE CLIMBS. 53 CLUB ACTIVITIES. 67 THE CONSTITUTION 69 HARVARD MOUNTAINEERING CLUB MEMBERSHIP, 1942-43 72 MT. SIR DONALD, B. C. (Showing famed N. IV. Arete in center. Summit, 10,818 feet) Photo, M. Miller Foreword HE Harvard Mountaineering Club is now emerging from its Club Officers T 19th year with as enviable a record of accomplishments as could possibly be hoped for in two decades of life. Ever since the first 1940-1941 1942 SUMMER organization meeting in September 1924 it has grown in prestige and tradition. This is no idle boast for with the coming and Pres. John Notman, '41 Pres. Andrew John Kauffman, Vice-Pres. John C. Cobb, '41 II, '43 going of expeditions every year there have been many successes. Sec. Andrew John Kauffman, II, '43 Vice-Pres. John P. Jewett, '43 How delighted we were to learn in 1936 of the great climb of those Treas. Maynard M. Miller, '43 Sec. Joseph T; Fitzpatrick, '45 six H.M.C. -

The United States Geological Survey in Alaska: Accomplishments During 1983
THE UNITED STATES GEOLOGICAL SURVEY IN ALASKA: - ACCOMPLISHMENTS DURING 1983 Susan Bartsch-Winkler and Katherine M. Reed, Editors U.S. GEOLOGICAL SURVEY CIRCULAR 945 Short papers describing results of recent geologic investigations DEPARTMENT OF THE INTERIOR DONALD PAUL HODEL, Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director .. - . - .. I Library of Congress Catalog Card Number 76-608093 Free on application to Distribution Branch, Text Products Section, U.S. Geological Survey, 604 South Pickett Street, Alexandria, VA 22304 CONTENTS Page mm........................................................................1 STATEWIDE Evidence that gold crystals can nucleate on bacterial spores, by John R. Watterson, James M. Nishi, and Theodore Botinelly ........... 1 Trace elants of placer gold, by Warren Yeend ........................................................ 4 N(I1THJ3N ALASKA Buried felsic plutons in Upper Ikvonian redbeds, central Brooks Range, by Donald J. Grybeck, John B. Cathrall, Jams R. LeCorrpte, and John W. Csdy. ................................... 8 New reference sect ion of the Noatak Sandstone, Nimiuktuk River, Misheguk hbuntain Quadrangle, central Brooks Range, by Tor H. Ni lsen, Wi l l iarn P. Brosgh, and J. Thornas l3.1 tro, Jr ........... 10 mcm ALASKA New radiametric evidence for the age and therml history of the mtamorphic rocks of the Ruby and Nixon Fork terranes, by John T. Dillon, WilliarnW. Patton, Jr., Sarmel B. Mukasa, George R. Tilton, Joel Blum, and Elizabeth J. W11 ..................... 13 Seacliff exposures of mtamrphosed carbonate and schist, northern Seward Peninsula, by Julie A. hmulin and Alison B. Till...,..,....... 18 Windy Creek and Crater Creek faults, Seward Peninsula, by Lbrrell S. Kaufmn ............................................... 22 Metarmrphic rocks in the western Iditarod Quadrangle, by hkrti L. Miller and Thorns K. -

The Official Magazine of The
OceTHE OFFICIALa MAGAZINEn ogOF THE OCEANOGRAPHYra SOCIETYphy CITATION Fenty, I., J.K. Willis, A. Khazendar, S. Dinardo, R. Forsberg, I. Fukumori, D. Holland, M. Jakobsson, D. Moller, J. Morison, A. Münchow, E. Rignot, M. Schodlok, A.F. Thompson, K. Tinto, M. Rutherford, and N. Trenholm. 2016. Oceans Melting Greenland: Early results from NASA’s ocean-ice mission in Greenland. Oceanography 29(4):72–83, https://doi.org/10.5670/oceanog.2016.100. DOI https://doi.org/10.5670/oceanog.2016.100 COPYRIGHT This article has been published in Oceanography, Volume 29, Number 4, a quarterly journal of The Oceanography Society. Copyright 2016 by The Oceanography Society. All rights reserved. USAGE Permission is granted to copy this article for use in teaching and research. Republication, systematic reproduction, or collective redistribution of any portion of this article by photocopy machine, reposting, or other means is permitted only with the approval of The Oceanography Society. Send all correspondence to: [email protected] or The Oceanography Society, PO Box 1931, Rockville, MD 20849-1931, USA. DOWNLOADED FROM HTTP://TOS.ORG/OCEANOGRAPHY SPECIAL ISSUE ON OCEAN-ICE INTERACTION Oceans Melting Greenland Early Results from NASA’s Ocean-Ice Mission in Greenland By Ian Fenty, Josh K. Willis, Ala Khazendar, Steven Dinardo, René Forsberg, Ichiro Fukumori, David Holland, Martin Jakobsson, Delwyn Moller, James Morison, Andreas Münchow, Eric Rignot, Michael Schodlok, Andrew F. Thompson, Kirsteen Tinto, Matthew Rutherford, and Nicole Trenholm Photo taken from Greenland's southwestern coast- line in July and August 2015 during Phase 1 of the TerraSond/Cape Race bathymetry survey. From https://omg.jpl.nasa.gov/portal/gallery/2015-survey-p1 72 Oceanography | Vol.29, No.4 ABSTRACT. -

Glacial Earthquakes and Glacier Seismicity in Greenland
Glacial Earthquakes and Glacier Seismicity in Greenland Stephen A. Veitch Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 © 2016 Stephen A. Veitch All rights reserved Abstract Glacial Earthquakes and Glacier Seismicity in Greenland Stephen A. Veitch The loss of ice from the Greenland ice sheet is an important contributor to current and future sea level rise occurring due to ongoing changes in the global climate. A significant portion of this ice mass loss comes through the calving of large icebergs at Greenland’s many marine-terminating outlet glaciers. However, the dynamics of calving at these glaciers is currently not well understood, complicating projections of future behaviour of these glaciers and mass loss from the Greenland ice sheet. The use of seismological tools has shown promise as a means of both monitoring and better understanding the dynamics of the calving process at these glaciers. On the global scale, data from the long-standing global seismic network has recorded the occurrence of glacial earthquakes, large long period earthquakes that occur dur- ing large calving events at near-grounded outlet glaciers. The occurrence and source parameters of these earthquakes provide insight into the link between glacier calv- ing and climatic and oceanic forcings, as well as information on the large-scale glacier-dynamic conditions under which these major calving events occur. On the more local scale, a deployment of seismometers around an individual glacier has provided insights on the seismic environment of a calving glacier, as well as the more immediate, short-term external drivers of calving events. -
Investigation of Coastal Dynamics of the Antarctic Ice
INVESTIGATION OF COASTAL DYNAMICS OF THE ANTARCTIC ICE SHEET USING SEQUENTIAL RADARSAT SAR IMAGES A Thesis by SHENG-JUNG TANG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE May 2007 Major Subject: Geography INVESTIGATION OF COASTAL DYNAMICS OF THE ANTARCTIC ICE SHEET USING SEQUENTIAL RADARSAT SAR IMAGES A Thesis by SHENG-JUNG TANG Submitted to the Office of Graduate Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Approved by: Chair of Committee, Hongxing Liu Committee Members, Andrew G. Klein John R. Giardino Head of Department, Douglas J. Sherman May 2007 Major Subject: Geography iii ABSTRACT Investigation of Coastal Dynamics of the Antarctic Ice Sheet Using Sequential Radarsat SAR Images. (May 2007) Sheng-Jung Tang, B.Eng., National Taiwan University of Science and Technology Chair of Advisory Committee: Dr. Hongxing Liu Increasing human activities have brought about a global warming trend, and cause global sea level rise. Investigations of variations in coastal margins of Antarctica and in the glacial dynamics of the Antarctic Ice Sheet provide useful diagnostic information for understanding and predicting sea level changes. This research investigates the coastal dynamics of the Antarctic Ice Sheet in terms of changes in the coastal margin and ice flow velocities. The primary methods used in this research include image segmentation based coastline extraction and image matching based velocity derivation. The image segmentation based coastline extraction method uses a modified adaptive thresholding algorithm to derive a high-resolution, complete coastline of Antarctica from 2000 orthorectified SAR images at the continental scale. -

Comparison of Four Calving Laws to Model Greenland Outlet Glaciers Youngmin Choi1, Mathieu Morlighem1, Michael Wood1, and Johannes H
Comparison of four calving laws to model Greenland outlet glaciers Youngmin Choi1, Mathieu Morlighem1, Michael Wood1, and Johannes H. Bondzio1 1University of California, Irvine, Department of Earth System Science, 3218 Croul Hall, Irvine, CA 92697-3100, USA Correspondence to: Youngmin Choi ([email protected]) Abstract. Calving is an important mechanism that controls the dynamics of marine terminating glaciers of Greenland. Ice- berg calving at the terminus affects the entire stress regime of outlet glaciers, which may lead to further retreat and ice flow acceleration. It is therefore critical to accurately parameterize calving in ice sheet models in order to improve the projections of ice sheet change over the coming decades and reduce the uncertainty in their contribution to sea level rise. Several calving 5 laws have been proposed, but most of them have been applied only to a specific region and have not been tested on other glaciers, while some others have only been implemented in one-dimensional flowline or vertical flowband models. Here, we test and compare several calving laws recently proposed in the literature using the Ice Sheet System Model (ISSM). We test these calving laws on nine tidewater glaciers of Greenland. We compare the modeled ice front evolution to the observed retreat from Landsat data collected over the past 10 years, and assess which calving law has better predictive abilities for each glacier. 10 Overall, the von Mises tensile stress calving law is more satisfactory than other laws for simulating observed ice front retreat, but new parameterizations that capture better the different modes of calving should be developed. -

Oceans Melting Greenland: Early Results from NASA's Ocean-Ice Mission in Greenland
Downloaded from orbit.dtu.dk on: Oct 01, 2021 Oceans Melting Greenland: Early Results from NASA's Ocean-Ice Mission in Greenland Fenty, Ian; Willis, Josh K.; Khazendar, Ala; Dinardo, Steven; Forsberg, René; Fukumori, Ichiro; Holland, David; Jakobsson, Martin; Moller, Delwyn; Morison, James Total number of authors: 17 Published in: Oceanography Link to article, DOI: 10.5670/oceanog.2016.100 Publication date: 2016 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Fenty, I., Willis, J. K., Khazendar, A., Dinardo, S., Forsberg, R., Fukumori, I., Holland, D., Jakobsson, M., Moller, D., Morison, J., Munchow, A., Rignot, E., Schodlok, M., Thompson, A. F., Tinto, K., Rutherford, M., & Trenholm, N. (2016). Oceans Melting Greenland: Early Results from NASA's Ocean-Ice Mission in Greenland. Oceanography, 29(4), 72-83. https://doi.org/10.5670/oceanog.2016.100 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Calving Event Detection by Observation of Seiche Effects on The
1 Calving event detection by observation of seiche effects on the 2 Greenland fjords 3 4 Fabian Walter1,2 5 Marco Olivieri3 6 John F. Clinton1 7 8 1Swiss Seismological Service, ETH Zürich, Switzerland 9 2Laboratory of Hydraulics, Hydrology and Glaciology, ETH Zürich, Switzerland 10 3Istituto Nazionale di Geofisica e Vulcanologia, Bologna, Italy 11 12 ABSTRACT 13 With mass loss from the Greenland ice sheet accelerating and spreading to higher latitudes, 14 the quantification of mass discharge in the form of icebergs has recently received much 15 scientific attention. Here, we make use of very low frequency (0.001‐0.01 Hz) seismic data 16 from three permanent broadband stations installed in the summers of 2009/2010 in 17 northwest Greenland in order to monitor local calving activity. At these frequencies, calving 18 seismograms are dominated by a tilt signal produced by local ground flexure in response to 19 fjord seiching generated by major iceberg calving events. A simple triggering algorithm is 20 proposed to detect calving events from large calving fronts with potentially no user 21 interaction. Our calving catalogue identifies spatial and temporal differences in calving 22 activity between Jakobshavn Isbræ and glaciers in the Uummannaq district some 200 km 23 further north. The Uummannaq glaciers show clear seasonal fluctuations in seiche‐based 24 calving detections as well as seiche amplitudes. In contrast, the detections at Jakobshavn 25 Isbræ show little seasonal variation, which may be evidence for an ongoing transition into 26 winter calving activity. The results offer further evidence that seismometers can provide 27 efficient and inexpensive monitoring of calving fronts. -

F L NAL RE PORT
Final report - mineral resources of northern Alaska Item Type Technical Report Authors Heiner, L.E.; Wolff, E.N. Citation Heiner, L.E. and Wolff, E.N., 1968, Final report - mineral resources of northern Alaska: University of Alaska Mineral Industry Research Laboratory Report No. 16, 306 p., 4 sheets. Publisher University of Alaska Mineral Industry Research Laboratory Download date 05/10/2021 08:34:04 Link to Item http://hdl.handle.net/11122/1065 F l NAL RE PORT MINERAL RESOURCES OF NORTHERN ALASKA M.I. R.L. Report No. 16 Submitted to the NORTH Commission Mineral Industry Research Laboratory University of Alaska Lawrence E. Heiner Ernest N. Wofff Editors University of Alaska Colorado State University June, 1968 Reprinted October, 1969 ACKNOWLEDGEMENTS It would be impossible to name all those who have contributed information and ideas to this report, through discussions, correspondence, or through access to unpublished material. The debt to those who have published information is acknowledged in the text and in the list of references in the back of the report. In addition, James A. Williams, Director of the State Division of Mine and Minerals, and Gordon Herreid of the same office, have given much of their time, allowed access to Division files, and provided working space in their office. Earl H. Beistline, Dean of the College of Earth Sciences and Mineral Industry, has helped greatly in expediting the work. George Grye, U. S. Geological Survey, has been most helpful in supplying information on Survey activities. C. L. Sainsbury and Peter 0. Sandvik have contributed ideas through discussions. Alan Doyle, Carl Heflinger, Douglas B.