Activities and Characteristics of Transfer Factors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Are Transfer Factors?
WHAT ARE TRANSFER FACTORS? More Next Blog» WHAT ARE TRANSFER FACTORS? Not a Vitamin, Mineral, Herb or Hormone. A NEW Nutritionparadigm & inHealthcare. Over 40 years in research,3000 studies in nations70 and hundreds of professionals are recommending thissupplement. product These are tiny molecules that transfer immunity informationentity from to oneanother, such as between a mother and her infant whobreastfeeds. she It does not interact with drug has It zerois alltoxity. natural,safe and effective for all people and all ages. About Me NAME: KYWONG LOCATION: KUALA LUMPUR, MALAYSIA View my complete profile SATURDAY, OCTOBER 21, 2006 WHAT ARE TRANSFER FACTORS? In a World WHAT ARE TRANSFER FACTORS? In a World Where Stres... Where Stress, Pollution, Poor Diet and New “Super Transfer Factor CARDIO bugs” Are Constantly Undermining People’s strengthened your heart wit... IMMUNE SYSTEM HERBS GINSENG & DONG QUAI - BREAST CANCER LINK? What are HOW TO BOAST UP & Transfer MODULATE YOUR IMMUNE SYSTEM AGA... Factors? In a New Trends - Fewer world where Breast Cancer Patients to stress, Get C... pollution, Acai berries in Transfer poor diet and Factor RioVida drinks new strains of What are Transfer Factors? In a world where “superbugs” str... are constantly undermining people’simmune systems, 4Life recognizes the importance of innovation in nutrition. We know that in order to January 2006 successfully battle these everyday challenges and to achieve overall well- February 2006 being, continual advancements need to be made to help support March 2006 immune strength and promote better health for you and your family. April 2006 May 2006 One of the strongest forces in nature for transferring and ensuring good October 2006 health, transfer factors has taken their place in the forefront of nutritional science. -

Characterization and Safety Profile of Transfer Factors Peptides, A
biomolecules Article Characterization and Safety Profile of Transfer Factors Peptides, a Nutritional Supplement for Immune System Regulation Hudson Polonini 1,* , Any Elisa de Souza Schmidt Gonçalves 2, Eli Dijkers 1 and Anderson de Oliveira Ferreira 1 1 Fagron B.V., Lichtenauerlaan 182, 3062 ME, Rotterdam, The Netherlands; [email protected] (E.D.); [email protected] (A.d.O.F.) 2 Infinity Pharma Brasil, Av. Pierre Simon de Laplace, 751 Techno Park, Campinas, SP 13069-320, Brazil; any.goncalves@infinitypharma.com.br * Correspondence: [email protected] Abstract: Imuno TF® is a nutritional supplement composed of isolated transfer factors (TF) from porcine spleen. It is composed of a specific mixture of molecules that impact functions of the biological systems and historically is linked to the immune system regulation. In this study, we demonstrate for the first time its proteomic analysis, nutritional composition, and safety profile in terms of mutagenic potential and acute oral dose (LD50). The obtained analysis indicated the product is a complex set of oligo- and polypeptides constituted of 163 different peptides which can potentially act on multiple mechanisms on the immune system pathways. The chemical composition showed low fat and low sugar content, saturated fatty acids-free, and the presence of 10 vitamins and 11 minerals. −1 No mutagenic effect was observed, and the LD50 was 5000 mg kg body weight. This accounts for a safe product to be used by the oral route, with potential benefits for the immune system. Citation: Polonini, H.; Gonçalves, Keywords: transfer factor; Imuno TF; safety profile; characterization; peptides; proteomics A.E.d.S.S.; Dijkers, E.; Ferreira, A.d.O. -

Structural Nature and Functions of Transfer Factors.Pdf
PDFlib PLOP: PDF Linearization, Optimization, Protection Page inserted by evaluation version www.pdflib.com – [email protected] Structural Nature and Functions of Transfer Factors CHARLES H. KIRKPATRICK Conrad D. Stephnzsmt Luburatmy for &starch in Immunology NationalJewish ckttcr for Immumhgy and Respimtmy Mdkine Denver, colorado80206 INTRODUCTION Successful transfer of cell-mediated immune responses from immune donors to nonimmune recipients was first described by Landsteiner and Chase in the early 1940~.~JGuinea pigs were sensitized to express contact allergy to picryl chloride or delayed hypersensitivity to tuberculin. After washed peritoneal exudate cells from the sensitized donors were given to unsensitized recipients, the recipients acquired the ability to express the cell-mediated immune responses of the donors. Further studies indicated that long-lasting and regularly successfid transfers were observed when there were syngeneic relationships between the donors and recipients, and only when intact, living donor cells were used. Attempts to transfer these reactivities with serum or dead cells were unsuccessful. Later experiments by Crepea and Cooke3 and Jeter and associates4 disclosed that under certain conditions it was possible to transfer contact hypersensitivity to simple chemicals such as poison ivy antigens and chlorodinitrobenzene (CDNB) from sensi- tized guinea pigs to unsensitized recipients with unliving lymphoid cells, allogeneic cells, and even cell extracts. The disparate results between these reports and the work of Chase have never been completely explained. The active component of the cell extracts was called “transfer factor,” and transfer of delayed-type hypersensitivity in humans with transfer factors was studied extensively by Lawrence and associates5in the 1950s. Their experiments involved lysates of blood leukocytes from donors who had positive delayed-hypersensitivity to antigens such as tuberculin (PPD), diphtheria toxoid, streptococcal M protein, or coccidioidin. -
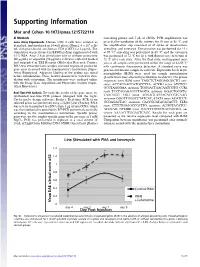
Supporting Information
Supporting Information Mor and Cohen 10.1073/pnas.1215722110 SI Methods containing primer and 5 μL of cDNA. PCR amplification was Gene Array Experiments. Human CD4 T cells were isolated as preceded by incubation of the mixture for 10 min at 95 °C, and described, and incubated in 24-well plates (Nunc), 4 × 106 cells/ the amplification step consisted of 45 cycles of denaturation, mL with plate-bound anti-human CD3 (OKT3) at 2 μg/mL. The annealing, and extension. Denaturation was performed for 15 s stimulation was performed in RPMI medium supplemented with at 95 °C; annealing was performed in 60 °C; and the extension 0.1% BSA. After 2 h of stimulation with or without cefuroxime was performed at 72 °C for 20 s, with fluorescence detection at (50 μg/mL) or ampicillin (50 μg/mL), cells were collected washed 72 °C after each cycle. After the final cycle, melting-point anal- and suspended in TRI Reagent (Molecular Research Center). yses of all samples were performed within the range of 62–95 °C RNA was extracted from samples and used to prepare probes for with continuous fluorescence detection. A standard curve was gene array in accord with the manufacturer’s instructions (Super- generated from one sample in each run. Expression levels of β2- Array Bioscience). Adequate labeling of the probes was tested microglobulin (B2M) were used for sample normalization before hybridization. Three healthy donors were tested in stim- (β-actin levels were affected by cefuroxime treatment). The primer ulation with cefuroxime. The membranes were analyzed online sequences were B2M sense TAGCTCTAGGAGGGCTG anti- with the Image Data Acquisition and Expression Analysis (Super- sense ACCACAACCATGCCTTA; ACVR2 sense ATCTCC- Array Bioscience). -

Chemokine Profiles of Interstitial Pneumonia in Patients With
www.nature.com/scientificreports OPEN Chemokine profiles of interstitial pneumonia in patients with dermatomyositis: a case control Received: 16 November 2016 Accepted: 30 March 2017 study Published: xx xx xxxx Katsuhiro Oda1, Takuya Kotani1, Tohru Takeuchi1, Takaaki Ishida1, Takeshi Shoda1, Kentaro Isoda1, Shuzo Yoshida1, Yasuichiro Nishimura2 & Shigeki Makino1 Chemokines play an important role in the pathophysiology of dermatomyositis (DM) with interstitial pneumonia (IP). However, the relation between chemokines and the disease activity or prognosis of DM-IP has not been elucidated. We evaluated the serum C-C motif chemokine ligand (CCL) 2, Th1 chemokines (C-X-C motif chemokine ligand [CXCL] 9, CXCL10, CXCL11), and Th2 chemokine (CCL17) profiles of 30 patients, and examined the relation between these chemokines and the disease activity or prognosis of DM-IP. Initial serum CCL2 level was higher in the death group (P = 0.007). To determine the cut-off points effective as poor prognostic factors of DM-IP, ROC curve analysis was carried out on initial serum CCL2 level. The value that maximized the area under the ROC curve was 894 pg/mL (sensitivity: 100%, specificity: 70.8%). Serum CCL2, CXCL9, CXCL10, and CXCL11 levels were lower at 2 weeks after treatment initiation than before treatment. Serum CCL2, CXCL10, and CXCL11 levels at 2 weeks after treatment initiation were higher in the death group. Serum levels of chemokines such as CCL2, CXCL10, and CXCL11 may be possible biomarkers of disease activity and prognosis in DM-IP, and serum CCL2 level may be useful when deciding initial treatment. Dermatomyositis (DM) is one of the idiopathic inflammatory myopathies characterized by inflammation of skin and muscle1, 2. -

Technical Paper
TECHNICAL PAPER BIOCHEMISTRY OF TRANSFER FACTOR I. INTRODUCTION Glutrasol products are comprised of three main ingredients: 1. transfer factor 2. glucans 3. lactic acid generating bacteria. Minor ingredients are adjusted for specific applications. This disclosure focuses on transfer factor. We know Glutrasol works because TEST groups repeatedly show dramatic improvement, relative to CONTROL groups. Here is information on how (biochemically) Glutrasol works. II. INDUCER/SUPPRESSOR FRACTIONS OF TRANSFER FACTOR Transfer factors are produced by T-lymphocytes and can transfer the ability to recognize a pathogen to cells that have not been in contact with the pathogen. They also heighten the immune system’s ability to react (increased reactivity or inducer function) to pathogens. Transfer factor produces a trigger for T-cell recognition of antigen. Transfer factor is a mixture of peptides, typically with a molecular weight < 10,000 Daltons. It's more appropriate to speak of "transfer factors" instead of "transfer factor." Fortunately, today's preparative methods produce consistent mixtures, which lead to repeatable responses. Fractions within the mixture serve different (and sometimes opposite) purposes. Two particularly important fractions are (1) the inducer fraction, and (2) the suppressor fraction [1][2]. Opposing functions were confirmed using the direct Leucocyte Migration Inhibition (LMI) test. These two fractions are consistent with field observations that transfer factor balances the immune system. For example, Date: 12/27/16 Author: Jack Menear, Chief Scientist and Patent Strategist, CortControl, LLC www.cortcontrol.com 1-855-982-2263 [email protected] TECHNICAL PAPER 1. The inducer fraction of transfer factor links the immune cells with an antigen-binding site, thereby increasing their reactivity to an antigenic stimulus. -

(12) United States Patent (10) Patent No.: US 7,867.484 B2 Samulski Et Al
USOO7867484B2 (12) United States Patent (10) Patent No.: US 7,867.484 B2 Samulski et al. (45) Date of Patent: Jan. 11, 2011 (54) HEPARIN AND HEPARAN SULFATE BINDING WO WO 2005,106046 A1 11/2005 CHMERIC VECTORS (75) Inventors: Richard Jude Samulski, Chapel Hill, OTHER PUBLICATIONS NC (US); Zhijian Wu, Chapel Hill, NC Rutledge et al. J. Virol. 72:309-319; 1998.* (US); Aravind Asokan, Chapel Hill, NC International Search Report and Written Opinion of International (US) Application No. PCT/US07/02251, mailed Sep. 18, 2008 (13 pages). Opie et al. “Identification of Amino Acid Residues in the Capsid (73) Assignee: University of North Carolina at Proteins of Adeno-Associated Virus Type 2 That Contribute to Chapel Hill, Chapel Hill, NC (US) Heparan Sulfate Proteoglycan Binding” Journal of Virology 77(12):6995-7006 (2003). (*) Notice: Subject to any disclaimer, the term of this Wu et al. “Mutational Analysis of the Adeno-Associated Virus Type patent is extended or adjusted under 35 2 (AAV2) Capsid Gene and Construction of AAV2 Vectors with U.S.C. 154(b) by 58 days. Altered Tropism” Journal of Virology 74(18):8635-8647 (2000). Grimm et al. “Helper Virus-Free, Optically Controllable, and Two (21) Appl. No.: 11/698,505 Plasmid-Based Production of Adeno-associated Virus Vectors of Serotypes 1 to 6’ Molecular Therapy 7(6): 839-850 (Jun. 2003). (22) Filed: Jan. 26, 2007 Grimm et al. “Preclinical in vivo evaluation of pseudotyped adeno associated virus vectors for liver gene therapy” Blood 102(7): 2412 (65) Prior Publication Data 2419 (Oct. 1, 2003). -

Evolution of COVID-19 Patients Treated with Immunoformulation, A
medRxiv preprint doi: https://doi.org/10.1101/2020.12.11.20246561; this version posted December 15, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC 4.0 International license . Evolution of COVID-19 patients treated with ImmunoFormulation, a combination of nutraceuticals to reduce symptomatology and improve prognosis: a multi-centred, retrospective cohort study Mariana Diaz Hernández1, Jully Urrea1, Luciano Bascoy2 1Clínica Arvila Magna. Avinguda Diagonal, 442, 08037 Barcelona, Spain. 2Clinic Bascoy. Carrer d'Horaci 9, 08022 Barcelona, Spain. Abstract Although a vast knowledge has already been gathered on the pathophysiology of COVID-19, there are still limited, non-optimal treatment options. In this paper, we describe a multicentre, retrospective, observational study to describe the course of SARS-CoV-2 disease in patients treated with ImmunoFormulation (IF), an add-on therapy developed to decrease duration of clinical symptoms. In parallel, a group of patients that did not receive IF was used for comparison (using standard of care treatment). A total of 39 patients were evaluated. Throughout the observational period, 90% of patients recovered in the IF cohort and 47.4% in the Control cohort (p=0.0057). From the symptoms with statistically significant differences, the duration of symptoms (i.e., the time to recover from it) was shorter in the IF cohort than in control cohort (in days, average), especially for fever (2.25 x 21.78), dry cough (4.38 x 24.00), dyspnoea (3.67 x 20.00), headache (2.00 x 26.50), diarrhoea (5.25 x 25.25), and weakness (1.92 x 23.30). -

Cpg and Transfer Factor Assembled on Nanoparticles Reduce Tumor Burden in Mice Glioma Cite This: RSC Adv.,2017,7, 11644 Model
RSC Advances View Article Online PAPER View Journal | View Issue CpG and transfer factor assembled on nanoparticles reduce tumor burden in mice glioma Cite this: RSC Adv.,2017,7, 11644 model Yi-Feng Miao,† Tao Lv,† Ran Wang, Hui Wu, Shao-Feng Yang, Jiong Dai and Xiao-Hua Zhang* This work describes the use of a transfer factor, a low molecular protein that can transfer cell mediated immunity from donor to recipient, and CpG, a clinically relevant toll-like receptor agonist, for treating glioma. Transfer factor and CpG were assembled onto gold nanoparticles via layer-by-layer assembly. The modified nanoparticles (i.e. particles assembled with transfer factor and CpG) were characterized by size, zeta potential, and loading. An in vivo tumor study revealed that the nanoparticles can inhibit tumor progression more effectively than using either TF or CpG alone or using an equivalent dose of CpG and TF in a soluble mixture. To investigate the anti-tumor mechanism, the modified nanoparticles were Creative Commons Attribution 3.0 Unported Licence. interacted with dendritic cells, macrophages. Viability tests showed that the modified nanoparticles did not affect cell viability, and neither did the use of soluble TF or CpG alone. Cell activation assessments showed that the modified nanoparticles can activate DC surface markers (CD80+, CD86+, and CD40+), and promote the production of cytokines (GM-CSF, TNF, and IL-6) from macrophages. In addition, the in Received 7th July 2016 vivo study revealed that the modified nanoparticles promoted the production of both inflammatory and Accepted 27th September 2016 effector cytokines in mice serum. -

The Mysterious, and Potentially Revolutionary, Immunological Properties of Transfer Factor: a Review
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 31 May 2019 doi:10.20944/preprints201905.0386.v1 1 The Mysterious, and Potentially Revolutionary, Immunological Properties of Transfer Factor: A Review Robert Root-Bernstein, Ph. D. Department of Physiology 2174 Biomedical and Physical Sciences Building Michigan State University East Lansing, MI 48824 USA [email protected] Abstract Transfer factor is the name given to material derived from activated lymphocytes that is probably composed of a complex of a peptide and a short segment of RNA and which has the reported ability to transfer specific T cell immunity to uncommitted lymphocytes. Many independent groups around the world reported isolating transfer factors between 1955 and 1990 and demonstrating their ability to transfer passive immunity from one animal or individual to another, often within 24 hours of inoculation. Such activity is potentially revolutionary both in making T cell vaccines readily manufacturable and also because the existence of transfer factors would undermine the basic assumptions of the clonal selection theory, which currently dominates immunological theory. Unfortunately, lack of the microanalytical and synthetic techniques required to properly identify transfer factors, combined with safety factors associated with it derivation from blood sources susceptible to HIV and prion infections, put an end to transfer factor research after 1990. This paper reviews the evidence supporting transfer factor activity and suggests that this potentially revolutionary -

Genetic Variants in ADAM33 Are Associated with Airway
Wang et al. BMC Pulmonary Medicine 2014, 14:173 http://www.biomedcentral.com/1471-2466/14/173 RESEARCH ARTICLE Open Access Genetic variants in ADAM33 are associated with airway inflammation and lung function in COPD Xinyan Wang1†, Wan Li2†, Kun Huang1, Xiaowen Kang1, Zhaoguo Li1, Chengcheng Yang1, Xiaomei Wu1* and Lina Chen2* Abstract Background: Genetic factors play a role in the development and severity of chronic obstructive pulmonary disease (COPD). The pathogenesis of COPD is a multifactorial process including an inflammatory cell profile. Recent studies revealed that single nucleotide polymorphisms (SNPs) within ADAM33 increased the susceptibility to COPD through changing the airway inflammatory process and lung function. Methods: In this paper, we investigated associations of four polymorphisms (T1, T2, S2 and Q-1) of ADAM33 as well as their haplotypes with pulmonary function and airway inflammatory process in an East Asian population of patients with COPD. Results: We found that T1, T2 and Q-1 were significantly associated with the changes of pulmonary function and components of cells in sputum of COPD, and T1 and Q-1 were significantly associated with cytokines and mediators of inflammation in airway of COPD in recessive models. 10 haplotypes were significantly associated with transfer factor of the lung for carbon monoxide in the disease state, 4 haplotypes were significantly associated with forced expiratory volume in one second, and other haplotypes were associated with airway inflammation. Conclusions: We confirmed for the first time that ADAM33 was involved in the pathogenesis of COPD by affecting airway inflammation and immune response in an East Asian population. Our results made the genetic background of COPD, a common and disabling disease, more apparent, which would supply genetic support for the study of the mechanism, classification and treatment for this disease. -

Profiling Serum Biomarkers in Patients with COPD
595 CHRONIC OBSTRUCTIVE PULMONARY DISEASE Thorax: first published as 10.1136/thx.2006.064428 on 13 March 2007. Downloaded from Profiling serum biomarkers in patients with COPD: associations with clinical parameters Victor Pinto-Plata, John Toso, Kwan Lee, Daniel Park, John Bilello, Hana Mullerova, Mary M De Souza, Rupert Vessey, Bartolome Celli ................................................................................................................................... Thorax 2007;62:595–601. doi: 10.1136/thx.2006.064428 Background: Chronic obstructive pulmonary disease (COPD) is an inflammatory lung disease associated with significant systemic consequences. Recognition of the systemic manifestations has stimulated interest in identifying circulating biomarkers in these patients. A systematic analysis was undertaken of multiple protein analytes in the serum of well characterised patients with COPD and matched controls using novel protein See end of article for authors’ affiliations microarray platform (PMP) technology. ........................ Methods: Forty-eight patients (65% men) with COPD (forced expiratory volume in 1 s ,55%) and 48 matched controls were studied. Anthropometric parameters, pulmonary function tests, 6-minute walk Correspondence to: Dr Bartolome R Celli, Caritas distance, the BODE index and the number of exacerbations were measured and the association of these St Elizabeth’s Medical outcomes with the baseline levels of 143 serum biomarkers measured by PMP was explored. Center, 736 Cambridge Results: Thirty biomarker clusters were identified and ranked by computing the predictive value of each cluster Street, Boston, Massachusetts 02135, USA; for COPD (partial least squares discriminant analysis). From the 19 best predictive clusters, 2–3 biomarkers [email protected] were selected based on their pathophysiological profile (chemoattractants, inflammation, tissue destruction and repair) and the statistical significance of their relationship with clinically important end points was tested.