A Feasibility Study to Investigate the Clinical Application of Functional
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Getting to the Western General Hospital
gettinggetting toto TheThe WesternWestern GeneralGeneral HospitalHospital atat CreweCrewe RoadRoad SthSth University Hospitals Division How to find your way around The following floor markings will help you find your way. I Junction of the North Corridor and the Link Corridor G Junction of the Link Corridor with the South Corridor L Junction of the South Corridor with the West Corridor AREA FLOOR BUILDING Oncology Outpatients.........................................Ground Floor ..............Edinburgh Cancer Centre Acute Receiving Unit ..........................................Ground Floor ..............Outpatient Building Outpatient Clinics (A,B,D)..................................1st Floor.....................Outpatient Building Body Scanner.......................................................Lower Ground.............Outpatient Building Outpatient Clinics................................................Lower Ground.............Anne Ferguson Bldg Breast Unit .............................................................................................As shown on the map Paediatric Clinics .................................................Lower Ground.............Outpatient Building Cardiac Lab Appointments .................................Ground Floor ..............Anne Ferguson Bldg Pathology.............................................................1st Floor.....................Alexander Donald Bldg Cardiology Day Unit ...........................................Ground Floor ..............Anne Ferguson Bldg Pharmacy..............................................................Ground -

Diabetes and Endocrinology
Diabetes and Endocrinology Specialty Induction Guide OPD2 Royal Infirmary of Edinburgh 51, Little France Crescent Edinburgh EH16 4SA Metabolic Unit Anne Ferguson Building Western General Hospital Crewe Road Edinburgh EH4 2XU July 2017 Induction information July 2017 Updated July 2017 Page 0 ENDOCRINOLOGY AND DIABETES IN EDINBURGH CONTENTS Contents ……………………………………………... Page 1 Introduction ………………………………………….. Page 2 RIE Who’s Who ……………………………….. Page 3-4 Unit meetings ……………………………... Page 4 Clinic timetable …………………………… Page 4-6 OPD2 information ………………………… Page 7 Diabetes clinic referral forms …………….... Page 7-8 Information for ST trainees…..…………...... Page 9 Roodlands Hospital Haddington.…………. Page 11 GP placement information ………………… Page 11 WGH Who’s Who ……………………………….... Page 12 Unit meetings ………………………………. Page 13 Clinic timetable …………………………….. Page 13-15 Information for ST trainees……………........ Page 16-18 Leith Community Treatment Centre ………. Page 18 GP placement information ………………… Page 19 CMT information …………………………... Page 19 General information SCI-Diabetes ……………………………….. Page 20-26 Diasend .......................................................... Page 27-31 CSII downloads on Carelink ......................... Page 32 ECED website and resources for T1DM ...... Page 33 Mental Health …………………………........ Page 34-35 IT systems for logging on call work ………. Page 36-37 Clinical outcoming ………………………… Page 38-40 Confidentiality and safe Email transmission . Page 41-43 Social media policy ………………………… Page 44-49 Computer Network Access application -
Group B 09-10
Supervisor Supervisor Address Supervisor Email Project Title Room S1642, RIE, 51 A systematic review of Little France Crescent, cognitive impairment in Dr Gillian Mead Edinburgh [email protected] patients with atrial Cardiology, RIE, 51 The Role of Endothelial Little France Cres, Progenitor Cells in Prof David Newby Edinburgh [email protected] Abdominal Aortic Dept of Cardiology, Diagnosis and Dr Muhammad Royal Hospital for Sick [email protected] Management of Walayat Children, Edinburgh, hs.uk Cardiomyopathy Endocrinology Unit, Centre for Cardiovascular Science, Queen's Effects of obesity in Dr Mandy Drake Medical Research [email protected] pregnancy Room S1642, RIE, 51 Post-stroke fatigue: the Dr Gillian Mead Little France Cres, [email protected] patient's prespective Dept of Urology, Optimisation of novel Western General markers of hypoxia in Mr Grant Stewart Hospital, Crewe Road, [email protected] prostate cancer Cardiology, RIE, 51 Pulse wave velocity Dr Nick Boon Little France Cres, [email protected] analysis Room E2.46, Centre for Changes in Inflammation Research, microvasculature Queen's Medical associated with chronic Research Institute, 47 graft injury following renal Ms Lorna Marson Little France Crescent, [email protected] transplantation Public Health Sciences, International child health Prof Harry Campbell Teviot Place, Edinburgh [email protected] development Division of Clinical A Systematic Review of Neurosciences, the effects of genetic Western General polymorphisms on the Dr Cathie Sudlow Hospital, Crewe Road, [email protected] outcome of stroke Community Child An audit of health services Dr Patricia D. -

A Brief Look at the History of the Deaconess Hospital, Edinburgh
J R Coll Physicians Edinb 2018; 48: 78–84 | doi: 10.4997/JRCPE.2018.118 PAPER A brief look at the history of the Deaconess Hospital, Edinburgh, 1894–1990 HistoryER McNeill1, D Wright2, AK Demetriades 3& Humanities The Deaconess Hospital, Edinburgh, opened in 1894 and was the rst Correspondence to: establishment of its kind in the UK, maintained and wholly funded as it E McNeill Abstract was by the Reformed Church. Through its 96-year lifetime it changed and Chancellor’s Building evolved to time and circumstance. It was a school: for the training of nurses 48 Little France Crescent and deaconesses who took their practical skills all over the world. It was a Edinburgh EH16 45B sanctum: for the sick-poor before the NHS. It was a subsidiary: for the bigger UK hospitals of Edinburgh after amalgamation into the NHS. It was a specialised centre: as the Urology Department in Edinburgh and the Scottish Lithotripter centre. And now it is currently Email: student accommodation. There is no single source to account for its history. Through the use [email protected] of original material made available by the Lothian Health Services Archives – including Church of Scotland publications, patient records, a doctor’s casebook and annual reports – we review its conception, purpose, development and running; its fate on joining the NHS, its identity in the latter years and nally its closure. Keywords: Charteris, Church of Scotland, Deaconess Hospital, Pleasance Declaration of interests: No confl ict of interests declared Introduction Figure 1 Charteris Memorial Church, St Ninian’s and the Deaconess Hospital, 1944 On a November morning in 1888, two men stood in the Pleasance area of Edinburgh looking across the street to an old house, which 200 years before had been the town residence of Lord Carnegie. -

Peter Goes Back to the Floor
THE NEWSPAPER FOR NHS LOTHIAN STAFF MAY/JUNE 2008 ISSUE 26 ConnectionsYOUR AWARD-WINNING NEWSPAPER PETER GOES BACK WIN A FABULOUS TO THE FLOOR PAMPER SESSION! PAGE 14 PAGE 15 £8M CASH BOOST FOR PUBLIC HEALTH SERVICES Government cash will help board tackle alcohol and tobacco misuse NHS Lothian has been awarded dependent drinkers, more treatment £8 million to deal with specific and prevention/education services public health issues such as and appropriate recording systems. alcohol misuse, sexual health The second largest sum of THANK YOU: auxiliary nurse Fay Watt, left, has a very special reason for presenting and stopping smoking money has been awarded for flowers to her nursing colleagues – turn to page 3 to read their heart-warming tale… programmes. prevention of blood-borne viruses. Just over £3.4m has been A continuing £2.52m is to be used awarded for alcohol misuse. to reduce the spread of HIV and The Scottish Government has hepatitis. tobacco control efforts. The Scottish Government acknowledges increased alcohol misuse funding Revised performance This being the case, funding by almost 150 per cent compared management arrangements to of £911,000 is being continued for that very significant progress has to 2007/08. ensure NHS boards use the NHS Lothian to provide stop been made in reducing smoking through The additional money is to help money effectively will be provided smoking services that help NHS boards meet targets for by the end of May. people quit. comprehensive tobacco control reducing consumption and related The Scottish Government The money allocated is to go harm and the Government expects acknowledges that very towards reducing smoking in help improve the sexual programme, which tackles considerable work to be done in significant progress has been made the adult population to 22 per health of Lothian’s population, health inequalities that are this area. -

Work Experience Application Form
For School Use If applicable: Work Experience Application Form IF APPLYING AS PART OF YOUR SCHOOL WORK EXPERIENCE WEEK PLEASE ENSURE YOU COMPLETE THIS SECTION: Work Experience Week/Dates........................................................................................... School....……………………………………………………………………………………………. Work Experience Coordinator.............................................Email............................................... School Telephone Number.................................................... IF NOT APPLYING AS PART OF YOUR SCHOOL WORK EXPERIENCE WEEK PLEASE ENSURE YOU COMPLETE THIS SECTION: Work Experience Week/Dates being requested............................................................................................. PERSONAL INFORMATION Title (Miss/Ms/Mrs/Mr)....................................................................................................... Name …………………………………………………………………………………………… Date of Birth ……………………………………………………… Age.................. Telephone Number ………………………………………………………………… Email Address ……………………………………………………………………… Address ……………………………………………………………………………… ………………………………………………………………………………………… Post Code …………………………… 1 PLEASE INDICATE YOUR CHOSEN CAREER PATH …………………………………………………………………………………………. Please indicate your choice of placement and list your 1. preference in order (e.g. midwifery, cardiology) 2. 3. PLEASE INDICATE THE LOCATIONS YOU CAN CONSIDER Placement Location: using numbers (1 being your first choice, 2 being your second choice, etc) please mark all the areas that you could -

Referral to Hospital a Guide to Referral and Waiting Times
You have been referred to: __________________________________ Midlothian Health & Social Care Partnership Referral to hospital A guide to referral and waiting times This information sheet tells you about what Your appointment happens when your doctor makes a hospital You should be given seven days notice of your referral for you. It covers waiting times, appointment. changing appointments and who to contact for more information. What to do if you can’t make your Making a referral appointment or have a query If the doctor is referring you to hospital, Please contact the department, using the phone she or he will contact the relevant hospital number on your appointment letter, as soon as department shortly after they have seen you can. Then they can give your appointment to you. At this point, it is really important to someone else and agree a more suitable time with you. make sure that the practice has your current address and phone number, so Turning down appointments that we can pass on the correct details to Please remember that if you turn down two the hospital. reasonable offers of appointment, the hospital From this point onwards, the hospital will be dealing may send you back to your GP and your waiting directly with you, usually by letter. Make sure you time will start again. ask your doctor which department and which hospital your are being referred to in case you need Going on holiday? to get in touch with them later on. If you are waiting to hear about an appointment Waiting times and you are about to go on holiday, please let the The hospital department will try to make your hospital know when you will be away. -
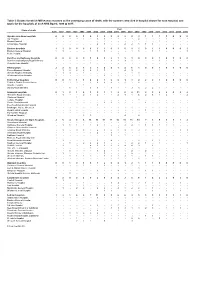
Table 3: Deaths for Which MRSA Was Recorded As the Underlying Cause Of
Table 3: Deaths for which MRSA was recorded as the underlying cause of death, with the numbers who died in hospital shown for each hospital, and totals for the hospitals of each NHS Board, 1996 to 2015 Year Place of death 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 Ayrshire and Arran hospitals 0 0 0 0 1 0 3 2 0 0 2 2 3 1 1 1 1 0 0 0 Ayr Hospital - - - - 1 - 1 1 - - - - 2 - - - 1 - - - Biggart Hospital - - - - - - - - - - - - - - - 1 - - - - Crosshouse Hospital - - - - - - 2 1 - - 2 2 1 1 1 - - - - - Borders hospitals 1 1 0 0 0 1 1 1 0 1 0 1 1 0 1 1 0 0 0 1 Borders General Hospital 1 1 - - - 1 1 1 - - - 1 1 - 1 1 - - - 1 Kelso Hospital - - - - - - - - - 1 - - - - - - - - - - Dumfries and Galloway hospitals 0 0 0 0 0 1 0 0 2 1 3 1 0 0 0 1 0 1 0 0 Dumfries and Galloway Royal Infirmary - - - - - - - - 2 1 3 1 - - - 1 - 1 - - Thomas Hope Hospital - - - - - 1 - - - - - - - - - - - - - - Fife hospitals 2 2 5 2 3 2 1 8 0 1 4 6 1 0 0 1 0 1 0 0 Queen Margaret Hospital - 1 2 2 2 2 1 7 - - 4 5 - - - 1 - - - - Victoria Hospital, Kirkcaldy 2 1 3 - 1 - - - - 1 - 1 1 - - - - 1 - - Whyteman's Brae Hospital - - - - - - - 1 - - - - - - - - - - - - Forth Valley hospitals 0 0 1 1 1 4 2 1 2 0 1 3 2 2 2 1 0 0 0 0 Falkirk & District Royal Infirmary - - - - - 1 1 1 1 - 1 - 1 - - - - - - - Sauchie Hospital - - - - 1 - - - - - - - - - - - - - - - Stirling Royal Infirmary - - 1 1 - 3 1 - 1 - - 3 1 2 2 1 - - - - Grampian hospitals 0 1 0 1 4 3 3 1 1 2 8 2 11 4 3 2 2 0 0 2 Aberdeen Royal Infirmary - 1 - - 2 1 2 -

Ellen's Glen House – Hawthorn Ward
Ellen’s Glen House – Hawthorn Ward Hawthorn ward is a 30 bedded complex clinical care ward which specialises in palliative and end of life care. We have two respite beds which enable us to provide support for families caring for their loved one at home. Our patient group can vary from someone in the late 40s to someone in their 90s. Patients are complex and have multiple co-morbidities ranging from Cancer, Stroke, Parkinsons Disease, MS and Diabetes to name a few. Hawthorn ward is located on the first floor of Ellen’s Glen House, which is part of Edinburgh Health and Social Care Partnerships community hospitals and is situated on Edinburgh’s Southside just off the Lasswade Road. It easily accessed by Bus or car and has its own onsite parking. We aspire to provide excellent, individualised care and actively encourage patients and their relatives to be involved in any decisions regarding their care. We strive to provide high quality person centred care for our patients and the team works very hard to achieve these goals. Unfortunately our patients tend to be with us for short periods of time, due to the nature of their illness. This makes it even more important for us as a team to ensure we give them a sense of belonging and worth, and help make meaningful contributions to decisions on their care and wellbeing. Hawthorn ward has a friendly and welcoming team environment. It is a great place to start off your Nursing career and equally we welcome staff with experience. There are plenty opportunities to enhance your knowledge and undertake further training ie: Flying start, National Procedural Award, extended roles. -

Lothian NHS Board Waverley Gate 2-4 Waterloo Place Edinburgh EH1 3EG
Lothian NHS Board Waverley Gate 2-4 Waterloo Place Edinburgh EH1 3EG Telephone: 0131 536 9000 www.nhslothian.scot.nhs.uk www.nhslothian.scot.nhs.uk Date: 12/06/2020 Your Ref: Our Ref: 4393 Enquiries to : Richard Mutch Extension: 35687 Direct Line: 0131 465 5687 [email protected] [email protected] Dear FREEDOM OF INFORMATION – BIKE RACKS I write in response to your request for information in relation to bike racks at NHS Lothian sites. I have been provided with information to help answer your request by the Estates Department of NHS Lothian. Question: Typical number staff employed at the site. Answer: Hospital Site Headcount THE ROYAL INFIRMARY OF EDINBURGH 6,597 THE WESTERN GENERAL HOSPITAL 4,420 ST JOHNS HOSPITAL 2,814 ROYAL EDINBURGH HOSPITAL 1,833 THE ROYAL HOSPITAL FOR SICK CHILDREN 1,377 ASTLEY AINSLIE HOSPITAL 509 EAST LOTHIAN COMMUNITY HOSPITAL 400 ROODLANDS HOSPITAL 292 MUSSELBURGH PRIMARY CARE CENTRE 250 LIBERTON HOSPITAL 217 MIDLOTHIAN COMMUNITY HOSPITAL 185 HERDMANFLAT HOSPITAL 149 THE PRINCESS ALEXANDRA EYE PAVILION 137 ROYAL VICTORIA HOSPITAL 132 BELHAVEN HOSPITAL 58 EDENHALL HOSPITAL 39 ST MICHAELS HOSPITAL 36 EDINGTON HOSPITAL 18 CORSTORPHINE HOSPITAL 6 Bike Racks - June 2020 Question: The number of bike racks at the site. The number of bike racks at the site which are in enclosed, locked compounds. Answer: Bake Enclosed Hospital Site Racks Racks THE ROYAL INFIRMARY OF EDINBURGH 237 32 THE WESTERN GENERAL HOSPITAL 440 168 ST JOHNS HOSPITAL 97 27 ROYAL EDINBURGH HOSPITAL 47 28 THE ROYAL HOSPITAL FOR SICK CHILDREN 24 0 RHCYP/DCN 20 0 ASTLEY AINSLIE HOSPITAL 60 0 EAST LOTHIAN COMMUNITY HOSPITAL 1 0 ROODLANDS HOSPITAL 0 0 MUSSELBURGH PRIMARY CARE CENTRE 6 0 LIBERTON HOSPITAL 3 0 MIDLOTHIAN COMMUNITY HOSPITAL 6 0 HERDMANFLAT HOSPITAL 0 0 THE PRINCESS ALEXANDRA EYE PAVILION 0 0 ROYAL VICTORIA HOSPITAL 0 0 BELHAVEN HOSPITAL 0 0 EDENHALL HOSPITAL 0 0 ST MICHAELS HOSPITAL 0 0 EDINGTON HOSPITAL 0 0 CORSTORPHINE HOSPITAL 0 0 I hope the information provided helps with your request. -

Introduction
Lothian Enteral Tube Feeding Best Practice Statement Introduction This document has been produced by the Lothian Enteral Tube Feeding Best Practice Statement Review Group. The group was established in May 2012 and devised a workplan. The remit of the Review Group was to update the Lothian Enteral Tube Feeding Best Practice Statement for Adults and Children that was published in December 2007. The content of the original document was reviewed, updated as necessary and new sections added to incorporate evidence since 2007. Where possible the recommendations are based on evidence, research or National Guidance. Where this was not available some statements are based on consensus of expert opinion and any related reference(s) listed. The Best Practice Statement applies to all adult, paediatric and neonate patients in Lothian, with the exception of the neonates in Simpson’s Reproductive Centre, Royal Infirmary of Edinburgh and St John’s Hospital. It is intended that this best practice statement should be implemented across NHS Lothian for hospitals and community. The Best Practice Statement will be reviewed on a rolling programme as required. At time of publication, some sections are currently under review, and this is clearly stated within the document. Julia Mackel Chair - Lothian Enteral Tube Feeding Best Practice Statement Review Group Review group membership Ruth Hymers Senior Dietitian Western General Hospital, Edinburgh Jenny Livingstone Senior Paediatric Dietician Royal Hospital for Sick Children, Edinburgh Julia Mackel (Chair) CENT -

Lothian NHS Board Waverley Gate 2-4 Waterloo Place Edinburgh EH1 3EG
Lothian NHS Board Waverley Gate 2-4 Waterloo Place Edinburgh EH1 3EG Telephone: 0131 536 9000 www.nhslothian.scot.nhs.uk Date: 30/12/2019 Our Ref: 4089 Enquiries to : Bryony Pillath Extension: 35676 Direct Line: 0131 465 5676 [email protected] Dear FREEDOM OF INFORMATION – CATERING I write in response to your request for information in relation to catering in NHS Lothian’s hospitals. Question: 1. What is the average cost per day in pound sterling allocated to feed an inpatient with non-exceptional dietary requirements staying in each of your hospitals for the last 5 years? For year please use calendar year up to 2019. For ‘hospitals’ please include sites where patients stay overnight on a ward and please ignore any sites that are self catering. Answer: Catering cost information is published for Scotland by the NHS National Services Scotland (NSS) Information Services Division (ISD) and can be found at the following weblinks: For 2019: https://www.isdscotland.org/Health-Topics/Finance/Costs/File-Listings-2019.asp For previous years: https://www.isdscotland.org/Health-Topics/Finance/Costs/costs- archive.asp Please click on the link, then download report R070-R110. Once the spreadsheet has opened look at tab R070. You can filter by health board; NHS Lothian is board code SSA20. Question: 2. Please list the hospital and care sites in your trust and state whether the catering service is outsourced and if outsourced please give the name of the company or companies this is currently outsourced to and the date of the outsourcing to this company.