CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
S.No. Shop Address 1 Anna Nagar Shanthi Colony
S.No. Shop Address Anna Nagar Shanthi Colony Aa-144, 2nd Floor, 3rd Avenue, (Next To Waves) Anna Nagar, Ch-600040. 1 Anna Nagar West No 670,Sarovar Building, School Road, Anna Nagar West, Chennai - 600101. 2 Mogappair East 4/491, Pari Salai, Mogappair East, (Near Tnsc Bank) Ch-600037 3 Mogappair West 1 Plot No.4, 1st Floor, Phase I, Nolambur,(Near Reliance Fresh) Mogappair 4 West, Ch-600037. Annanagar West Extn Plot No: R48, Door No - 157, Tvs Avenue Main Road,Anna Nagar West 5 Extension,Chennai - 600 101. Opp To Indian Overseas Bank. Red Hills 1/172a, Gnt Road, 2nd Floor, Redhills-Chennai:52. Above Lic, Next To Iyappan 6 Temple K.K.Nagar 2 No.455, R.K.Shanuganathan Road, K K Nagar, Land Mark:Near By K M 7 Hospital, Chennai - 600 078 Tiruthani No. 9, Chittoor Road, Thirutani - 631 209 8 Anna Nagar (Lounge) C Block, No. 70, Tvk Colony, Annanagar East, Chennai - 102. 9 K.K.Nagar 1 Plot No 1068, 1st Floor, Munuswami Salai, (Opp To Nilgiri Super Market) 10 K.K.Nagar West, Ch-600078. Alapakkam No. 21, 1st Floor, Srinivasa Nagar,Alapakkam Main 11 Road,Maduravoyal,Chennai 600095 Mogappair West 2 No-113, Vellalar Street, Mogappair West, Chennai -600 037. 12 Poonamalle # 35, Trunk Road, Opp To Grt Poonamalle Chennai-600056. 13 Karayanchavadi N0. 70, Trunk Road, Karayanchavadi, Poonamallee, Chennai - 56 14 Annanagar 6th Avenue 6th Avenue,Anna Nagar,Chennai 15 Chetpet Opp To Palimarhotel,73,Casamajorroad,Egmore,Ch-600008 16 Egmore Lounge 74/26,Fagunmansion,Groundfloor,Nearethirajcollege,Egmore,Chennai-600008 17 Nungambakkam W A-6, Gems Court, New.25 (Old No14), Khader Nawaz Khan Road, (Opp Wills 18 Life Style) Nungambakkam, Ch-600034. -
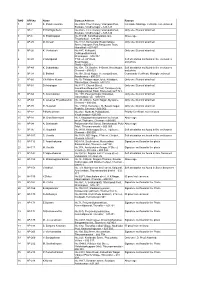
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

B29N Bus Time Schedule & Line Route
B29N bus time schedule & line map B29N Otteri View In Website Mode The B29N bus line (Otteri) has 4 routes. For regular weekdays, their operation hours are: (1) Otteri: 8:55 PM (2) Perambur B.S.: 10:00 AM (3) Periyar Nagar: 6:58 PM (4) Velachery: 8:10 AM - 5:28 PM Use the Moovit App to ƒnd the closest B29N bus station near you and ƒnd out when is the next B29N bus arriving. Direction: Otteri B29N bus Time Schedule 16 stops Otteri Route Timetable: VIEW LINE SCHEDULE Sunday 8:55 PM Monday 8:55 PM Periyar Nagar Tuesday 8:55 PM S.R.B Colony Wednesday 8:55 PM 70 Feet Road Thursday 8:55 PM Agaram Junction Friday 8:55 PM Kamarajar Salai Saturday 8:55 PM Veenus Chembium Old Post O∆ce B29N bus Info Lourde School Direction: Otteri Stops: 16 Trip Duration: 11 min Mangalapuri Line Summary: Periyar Nagar, S.R.B Colony, 70 Feet Chandra Yogi Samadhi Rd, Chennai Road, Agaram Junction, Kamarajar Salai, Veenus, Chembium Old Post O∆ce, Lourde School, Perambur Mangalapuri, Perambur, Jamalaya, Mettupalayam, Otteri Church, Binny Mills, Otteri Police Station, Otteri Jamalaya Mettupalayam Otteri Church Binny Mills Otteri Police Station Otteri Kancheepuram, Chennai Otteri Otteri Bridge, Chennai Direction: Perambur B.S. B29N bus Time Schedule 56 stops Perambur B.S. Route Timetable: VIEW LINE SCHEDULE Sunday 10:00 AM Monday 10:00 AM Velachery (Vijaynagar) Velachery Bypass Road, Chennai Tuesday 10:00 AM Velachery (Vijaynagar) Wednesday 10:00 AM Bus Way, Chennai Thursday 10:00 AM Dhandeswaram Friday 10:00 AM Thandeeswaram Saturday 10:00 AM Gandhi Nagar Gurunanak College Velachery Main Road, Chennai B29N bus Info Direction: Perambur B.S. -

India Cements Capital Ltd
09.09.2021 List containing names and address of ADs Cat-II licensed by Chennai Regional Office Name of the Regional Office – Chennai SL SL Name And Address Of AD Cat-II Address Of The Branch No. No. 1 PRITHVI EXCHANGE 1 No: 100, Ground Floor, L. B. Road, (INDIA)LIMITED (Dr. MuthulakshmiSalai), Adyar, Gee Gee Universal, 2nd Floor Chennai - 600 020 Door No.2, Mc Nichols Road 2 No: 135, S. M. Narayana Nagar, Chetpet Anna Nagar West Extension, Chennai - 600 031 (Near Madras Medical Mission Hospital) Chennai - 600 101 3 New No: 74, Old No: 20A, 1st Avenue, Dhanalakshmi Apartment, Ground Floor Ashok Nagar, Chennai – 600 083 4 First Floor, No.5/347 Rajiv Gandhi Salai (OMR) Okkiampettai Chennai - 600 097 5 Shop No: 9, Ground Floor, Taas Mahal No: 10, Montieth Road, Egmore Chennai - 600008 6 No: 97/3, Residency Road, Ground Floor, (Opp.to Bishop Cotton Girls School), Bengaluru - 560 025 7 Shop No. 4, Ground Floor, Lakshmi Venkateswara Arcade, No: 657-58, 11th Main Road, 4th Block, Jaya Nagar, Bengaluru - 560 011 8 Office No.208, 2nd Floor, Chinthamani Plaza, Mohan Studio Compound, AndheriKurla Road, Andheri (E), Mumbai – 400 099. 9 Unit No: 5 & 6, Ground Floor, Topaz Building,(OppOdessy), 63-883, Punjagutta, Hyderabad-500 082 10 Shop No.6, AVM Towers, Bombay High Way, Near Vishwanath Theatre, Kukkatpalli, R.R District, Hyderabad – 500 072 11 T.P.3, F.P.231/1 at 103 F.Floor Ambalal Avenue Stadium Five Cross Road Near Tushar Complex Off C G Road, Navrangapura Ahmedabad-380 009 12 No.66/4572, F3 First Floor, Emjee Square, M.G. -

Student Writing in the University of Madras: Traditions, Courses, Ambitions
CHAPTER 22. STUDENT WRITING IN THE UNIVERSITY OF MADRAS: TRADITIONS, COURSES, AMBITIONS By Susaimanickam Armstrong University of Madras (India) Writing courses and related initiatives at the University of Madras make available to the students the skills of writing. According to their programs and interests, students are trained in many forms of writing, including professional, creative, and research. This profile describes some techniques and assignments used as part of these writing oppor- tunities in various disciplines. It attempts to critically understand the role of the university in forwarding new trends in writing and com- munication that play a major role in establishing careers of students and that are shaping the development of the academic and creative world. The author describes in detail his own expanded uses of writing in a literature course. Further, the essay spells out the progress of “soft skills” programs in several languages, by which students gain new hori- zons in language acquisition. Finally, it projects a range of new writ- ing/communications initiatives by which the university can expand its importance in the burgeoning economy of its region. The 153-year old University of Madras (UOM) is the mother of almost all the old universities of Southern India. It is an affiliated, state university under the Government of Tamil Nadu. The university area of jurisdiction has been confined to three districts of Tamil Nadu in recent years. This is consequent to the establishment of various universities in the state and demarcation of the university territories. Through its long history, the university has diversified its teaching and re- search. -

Details of Assistant Public Information Officer, Public Information Officer
Details of Assistant Public Information Officer, Public Information Officer and Appellate Authorities appointed under Right to Information Act in respect of Chennai Collectorate and Taluk Offices Name of the Assistant Public Public Appellate Office Address Phone No. and Department Information Information Authority E mail ID Officer Officer Collectorate Huzur P.A(G) to District 62, Rajaji Salai, Chennai-1 (General) Sarishtadar Collector Revenue Ph.No. 25268320 (General) Officer [email protected] Excise Huzur Deputy Collector 62, Rajaji Salai, Chennai-1 Sarishtadar Commissioner Ph.No. 25270456 (Excise) (Excise) [email protected] Backward Superintendent District Collector 62, Rajaji Salai, Chennai-1 Classes Welfare (Senior) Backward Ph.No. 25241002 Classes and [email protected] Minorities Welfare Officer Adi Dravidar Superintendent District Adi- Collector 62, Rajaji Salai, Chennai-1 Welfare (Senior) dravidar and Ph.No. 25268320 Tribal Welfare [email protected] Officer Tondiarpet Zonal Deputy Head Quarters Tahsildar No.473, Thiruvoittyur High Road, (Opp. Taluk Tahsildar Deputy to Cholrea Hospital),Tondiarpet, Chennai-81 Tahsildar Ph.No. 25911727 [email protected] Purasawalkam Zonal Deputy Head Quarters Tahsildar No.3, Raja Muthiaiah Salai, (Old Fort- Taluk Tahsildar Deputy Tondiarpet Taluk Office) Purasawalkam, Chennai-3 (Near Nehru Indoor Stadium) Tahsildar Ph.No. 25388978 [email protected] Perambur Taluk Zonal Deputy Head Quarters Tahsildar No.3, Perambur High Road, Tahsildar Deputy Near Perambur Railway Station, Perambur, Chennai-600 011. Tahsildar Ph.No. 25375131 [email protected] Ayanavaram Zonal Deputy Head Quarters Tahsildar No.25, United India Colony first main Taluk Tahsildar Deputy Road, Ayanavaram, Chennai-600 023 (Near Ayanavaram Market) Tahsildar Ph.No. 26741726 [email protected] Egmore Taluk Zonal Deputy Head Quarters Tahsildar No.88, Mayor Ramanathan Salai, Tahsildar Deputy Chetpet, Chennai-600 031 (Near Palimer Hotel) Tahsildar Ph.No. -

Various Activities Under Environment Protection and Renewable Energy Development Fund –– Release of Funds – Orders – Issued
1 ABSTRACT Environment Control – Various Activities under Environment Protection and Renewable Energy Development Fund –– Release of funds – Orders – Issued. ------------------------------------------------------------------------------------------------- ENVIRONMENT AND FORESTS (EC2) DEPARTMENT G.O.(3D).No. 80 Dated :10.9.2014 ÂUtŸSt® M©L – 2045 #a, Mtâ -25 1) G.O.(3D) No.43, Environment and Forests (EC.2) Department, dated 19.7.2010. 2) G.O.(Ms) No.72, Environment and Forests (FR.6) Department, dated 24.06.2014 3) From the Director of Environment Letter No.1509/EMAT/2014, dated 28.8.2014. -------- ORDER:- In the Government order first read above, among other things, orders were issued constituting an Empowered Committee under the Chairmanship of Chief Secretary and Secretaries, Environment and Forests Department, Finance Department among others as members to direct/guide the operationalization of Environment Protection and Renewable Energy Development Fund to consider various proposals thereunder. 2. The Hon’ble Minister (Finance) in his budget speech 2014-15 among other things has announced the followings:- A sum of Rs.50 crore was allocated out of the Environment Protection and Renewable Energy Fund to various conservation activities, including Rs.12 crore for the restoration of the Chetpet Lake during 2013-2014. During 2014-2015, a sum of Rs.100 crore will be allocated for this fund. After meeting the balance requirement of Rs.30 crore for the Chetpet Lake restoration, the remaining funds will be used to restore lake, water bodies and for other works in Chennai and other Cities. 3. The Director of Environment in his letter 3rd read above has stated that the proposals received from various departments to implement under Environment Protection and Renewable Energy Development Fund for the 2 year 2014-2015 was placed before the Empowered Committee and the Empowered Committee has approved the following schemes :- Amount Sl. -

Dept. of Service Learning (Outreach) Annual Report 2017-2018
Loyola College (Autonomous), Chennai – 600 034. Dept. of Service Learning (Outreach) Annual Report 2017-2018 Introduction The Department of Service Learning (formerly called Outreach) was introduced in Loyola College during the academic year 2001-2002, with a view to make the students experience and express concern for the society, especially the disadvantaged sections. The idea of this extension programme is to make Loyola College students to commit themselves to create a better nation by getting involved in the neighbourhood community. With this spirit, the Service Learning unit works in 41 slums (urban villages or sub-standard settlements) around the college campus. The department has been collaborating with Chennai Corporation, slum clearance board, the corporation schools and like-minded NGOs and institutions in the neighbourhood ever since its inception. To make the initiative a reality, the second year undergraduate students from all departments of both Shift I and II, numbering to about1850 students are involved in various activities. The activities are streamlined and focused on General Health, Children, Youth, Women, Disabled and Elders, and other concerns of our times. They visit the slums two days per week. Vision: To make the students socially responsible citizens who are sensitive to the needs of disadvantaged sections. Values: With love and concern for others, students should dedicate themselves to community service, thereby making them men and women for others. Mission: To create a society with committed youth to promote education, health and environment for the less privileged. Goal: We hope to bring about social, cultural and economic empowerment in the neighbourhood communities for a sustainable and positive change. -

29B Bus Time Schedule & Line Route
29B bus time schedule & line map 29B Perambur B.S - Saidapet View In Website Mode The 29B bus line (Perambur B.S - Saidapet) has 2 routes. For regular weekdays, their operation hours are: (1) Perambur B.S.: 6:17 AM - 8:10 PM (2) Saidapet: 5:15 AM - 6:45 PM Use the Moovit App to ƒnd the closest 29B bus station near you and ƒnd out when is the next 29B bus arriving. Direction: Perambur B.S. 29B bus Time Schedule 29 stops Perambur B.S. Route Timetable: VIEW LINE SCHEDULE Sunday 6:17 AM - 8:10 PM Monday 6:17 AM - 8:10 PM Saidapet Tuesday 6:17 AM - 8:10 PM Thadandar Nagar Wednesday 6:17 AM - 8:10 PM Cit Nagar Thursday 6:17 AM - 8:10 PM Thiagaraya Nagar Friday 6:17 AM - 8:10 PM Panagal Park Saturday 6:17 AM - 8:10 PM Pondy Bazaar Raja Badar Street, Chennai Vanavali 29B bus Info Direction: Perambur B.S. Teynampet Stops: 29 Trip Duration: 35 min Anna Salai Bus Lane, Chennai Line Summary: Saidapet, Thadandar Nagar, Cit D.M.S.Metro Station Nagar, Thiagaraya Nagar, Panagal Park, Pondy Bazaar, Vanavali, Teynampet, D.M.S.Metro Station, Brs Hospital, Valluvar Kottam, Corporation Brs Hospital School/O∆ce, Sterling Market, Sterling Road, Chetpet, Kmc, Neyveli House, Purasawalkkam, Valluvar Kottam Doveton, Vasanthi Theatre, Kutty Street, Pattalam, Dasamahan, Dasamahan, Otteri, Binny Mill Canteen, Corporation School/O∆ce Otteri Church, Jamalaya, Perambur Sterling Market Sterling Road Chetpet Kmc Neyveli House EVR Periyar Salai (Poonamallee High Road), Chennai Purasawalkkam Doveton Doveton Flyover, Chennai Vasanthi Theatre Kutty Street Pattalam Dasamahan -

India COVID-19 Hospitals and Test Centres
1) NON NETWORK HOSPITALS Sr. Name of Hospital Address City PIN No. Plot No. 3&4, 1 Narayana Health Group Sadaramangala Bangalore 560066 Industrial Area 2 NH MMI Narayana Superspeciality Hospital - Raipur Dhamtari road, lalpur Raipur 492001 A Block, Near Kela 3 Fortis Hospital Shalimar Bagh Godown, NEW DELHI, New Delhi 110088 DELHI, Mulund Goregaon Link 4 Fortis Hospitals Ltd - Mulund Road, MUMBAI, Mumbai 400078 MAHARASHTRA, On Kalyan -Shill Road, 5 Fortis Hospitals Ltd - kalyan Thane 421301 Kalyan 5 th floor, mini seashore Navi 6 Hiranandani Hospital (Fortis) road, sector-10, Vashi, 400703 Mumbai Navi Mumbai CA#8, Ideal Homes 7 SSNMC Super Speciality Hospital Bangalore 560098 Township, 8 Apollo Hospitals Sarita Vihar, NEW DELHI New Delhi 110076 Block J, Mayfield 9 CK Birla Hospital For Women-Gurgaon Gurgaon 122018 Garden, Sector 51 Mulund Goregaon Link Fortis Hospital (Telegram Channel - Fortis Mental 10 Road, MUMBAI, Mumbai 400078 Health) MAHARASHTRA, 11 Manipal Hospital 98, HAL Airport Road, Bangalore 560017 Dr Baba Saheb Bharat Ratna Dr Babasaheb Ambedkar Memorial 12 Ambedkar Road, Byculla Mumbai 400012 Hospital - Byculla East, Mumbai, M M Marg, RBI Staff 13 Jagjivan Ram Hospital - Mumbai Colony, Mumbai Mumbai 400008 Central, L M Nadkarni Marg, 14 Mumbai Port Trust Hospital - Wadala Mumbai 400037 Wadala East, Veera Desai Road, 15 Andheri Sports Complex - Mumbai Mumbai 400053 Andheri West New Mhada Colony, Municipal Capacity Building and Research (MCMCR) in 16 Savarkar Nagar, Mumbai 400076 Powai Chandivali, Powai, Taharpur Rd, Taharpur, 17 Rajeev Gandhi Super Speciality Hospital Taharpur Village, New Delhi 110093 Dilshad Garden, Tahirpur Rd, GTB 18 GTBH (Guru Teg Bahadur Hospital) Enclave, Dilshad New Delhi 110095 Garden, Jawahar Lal Nehru 19 LNH (Lok Nayak Hospital) New Delhi 110002 Marg, Central, Maulana Azad Medical College Campus, 20 LNH (MAIDS) New Delhi 110002 Bahadur Shah Zafar Marg, Metro Station, Bhagawan Mahavir 21 Dr. -

Transport Department
Transport Department 101 – Ennore (Professor In-Charge:Ms. J.Shanmuga Kani-9941314199) (Via:Thiruvottiyur, Beach Station, KMC, Rohini) Thalankuppam beach(5.20am)-S.V.M nagar(5.23am)-kamarajar BS(5.27am)-Ennore BS(5.30am)- Ernavur(5.35am)-liftgate(5.38am)-WIMCO(5.40am)-AJAX(5.43am)-Periyar Nagar(5.46am)-Sugam Hospital(5.48am)-Thiruvotriyur Market(5.50am)-Theradi(5.52am) -Rajakadai police station (5.57am)- Rajakadai BS(6.00am)- Thangal(6.02am)- Dental(7.00am)-SEC(7.30am) 102 – Tollgate(Professor In-Charge: Mr.J.Jeya Caleb - 9790719303) (Via:Clock Tower, Waltax Road, Rohini) ITC(5.35 am) - Ajax (5.40 am) - Thiruvottiyur Market (5.45 am) - Ellaiamman Koil (5.50am) - Tollgate(6.00 am)-Tondiarpet Lakshmi Koil(6.10 am)-Apollo(6.15 am)-Clock Tower(6.17am) -Post Office(6.19am)-Singapore Shoppe(6.21am)-Pandian Theatre(6.23am)- RSRM(6.28am)-Parrys (6.32 am) - Dental(7.00 am)- SEC (7.30 am) 103 – Royapuram(Professor In-Charge:Ms.S.Chitra - 9962616646) (Via:Kasimedu, Beach Station, Central, Rohini) Burma Nagar(5.45am) – ITC(5.47)-Ellaimman Koil(5.52am)-Lakshmi Koil Beach Road(6.05am) – Mathivanan Office(6.10am) – Kasimedu(6.12am) – Kalmandapam(6.15am) – Clive Factory(6.20am)- Beach Station(6.23am)- Dasprakash(6.35am) - Dental(7.00am)-SEC(7.30am) 104 – IOC(Professor In-Charge: Mr.R.Jagadeshwaran–9566127991) (Via:Power House, Apollo, Mint,Veppery PS) IOC(6.05am)-Power House(6.08am)-Maharani(6.20am)-Pencil Factory(6.24am)- Mint(6.27am)-Vepery Police Station(6.35am) - Dental(7.00am)-SEC(7.30am) 105 – Tondiarpet(Professor In-Charge: Mr.D.Lingaraja-9176108306) -

Hospital Name Address
SLNO ZONE WARD HOSPITAL NAME ADDRESS 1 01-Thiruvottyur 001 SUMITHIRA CLINIC K.H. ROAD KATHIVAKKAM THALANKUPPAM 600057 2 01-Thiruvottyur 001 URBAN HEALTHPOST KAMARAJ NAGAR KATHIVAKKAM KAMARAJ NAGAR 600057 3 01-Thiruvottyur 001 SRI VEGNESH CLINIC C-15,THLANKUPPAM MAIN ROAD KATHIVAKKAM ULAGANATHAPURAM 600057 4 01-Thiruvottyur 001 S.S. CLINIC 6,6TH STREET KATHIVAKKAM ULAGANATHAPURAM 600057 5 01-Thiruvottyur 001 JAI CLINIC 3,7TH STREET ULAGANATHAPURAM THALANKUPPAM 600057 6 01-Thiruvottyur 001 JAI ASHWIN CLINIC 4, SATHYAVANI MUTHU NAGAR 7TH STREET, ENNORE, CHENNAI 600057 7 01-Thiruvottyur 001 SRI VEGNESH CLINIC C-15,THALANKUPPAM MAIN ROAD KATHIVAKKAM ULAGANATHAPURAM 600057 8 01-Thiruvottyur 002 RAJA CLINIC GIRIYAPPATHOTTAM KATHIVAKKAM SIVANPADAI VEEDHI 600057 9 01-Thiruvottyur 002 SRI VENUGOPAL CLINIC R.S. ROAD KATHIVAKKAM VALLUVAR NAGAR 600057 10 01-Thiruvottyur 002 SRI VENKATESWARA CLINIC K.H. ROAD KATHIVAKKAM SANJAIGANDHI NAGAR 600057 11 01-Thiruvottyur 002 THANGAMMAL CLINIC K.H. ROAD KATHIVAKKAM KATTUKUPPAM 600057 12 01-Thiruvottyur 002 MATERNITY CENTRE VILLAGE STREET KATHIVAKKAM SIVANPADAI VEEDHI 600057 13 01-Thiruvottyur 002 VIJAYA CLINIC THLANKUPPAM KATHIVAKKAM THALANKUPPAM 600057 14 01-Thiruvottyur 002 IPP-V HOSPITAL 03/592,K.H. ROAD KATHIVAKKAM K.H.ROAD 600057 15 01-Thiruvottyur 002 IPP-V HOSPITAL KAMARAJ NAGAR 5TH STREET KATHIVAKKAM KAMARAJ NAGAR 600057 16 01-Thiruvottyur 002 VIJAYA CLINIC GANDHI NAGAR KATHIVAKKAM GANDHI NAGAR 600057 17 01-Thiruvottyur 004 SELVA CLINIC & MATERNITY CENTR 72,KAMARAJ NAGAR THIRUVOTTIYUR ERNAVOOR 600019