Memantine Prevents the WIN 55212-2 Evoked Cross
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Role of the Endocannabinoid System and Medical Cannabis
Brigham Young University BYU ScholarsArchive Student Works 2016-12-19 Role of the Endocannabinoid System and Medical Cannabis Sabrina Jarvis Brigham Young University, [email protected] Sean Rasmussen Brigham Young University, [email protected] Blaine Winters Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/studentpub Part of the Nursing Commons The College of Nursing showcases some of our best evidence based scholarly papers from graduate students in the Family Nurse Practitioner Program. The papers address relevant clinical problems for advance practice nurses and are based on the best evidence available. Using a systematic approach students critically analyze and synthesize the research studies to determine the strength of the evidence regarding the clinical problem. Based on the findings, recommendations are made for clinical practice. The papers are published in professional journals and presented at professional meetings. BYU ScholarsArchive Citation Jarvis, Sabrina; Rasmussen, Sean; and Winters, Blaine, "Role of the Endocannabinoid System and Medical Cannabis" (2016). Student Works. 192. https://scholarsarchive.byu.edu/studentpub/192 This Master's Project is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Student Works by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Role of the Endocannabinoid System and Medical Cannabis Sean I. Rasmussen An evidence based scholarly paper submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Masters of Science Sabrina Jarvis, Chair Blaine Winters College of Nursing Brigham Young University Copyright © 2016 Sean I. -

Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back
International Journal of Molecular Sciences Review Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back Osnat Almogi-Hazan * and Reuven Or Laboratory of Immunotherapy and Bone Marrow Transplantation, Hadassah Medical Center, The Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem 91120, Israel; [email protected] * Correspondence: [email protected] Received: 21 May 2020; Accepted: 19 June 2020; Published: 23 June 2020 Abstract: The Cannabis plant contains numerous components, including cannabinoids and other active molecules. The phyto-cannabinoid activity is mediated by the endocannabinoid system. Cannabinoids affect the nervous system and play significant roles in the regulation of the immune system. While Cannabis is not yet registered as a drug, the potential of cannabinoid-based medicines for the treatment of various conditions has led many countries to authorize their clinical use. However, the data from basic and medical research dedicated to medical Cannabis is currently limited. A variety of pathological conditions involve dysregulation of the immune system. For example, in cancer, immune surveillance and cancer immuno-editing result in immune tolerance. On the other hand, in autoimmune diseases increased immune activity causes tissue damage. Immuno-modulating therapies can regulate the immune system and therefore the immune-regulatory properties of cannabinoids, suggest their use in the therapy of immune related disorders. In this contemporary review, we discuss the roles of the endocannabinoid system in immunity and explore the emerging data about the effects of cannabinoids on the immune response in different pathologies. In addition, we discuss the complexities of using cannabinoid-based treatments in each of these conditions. -

Endocannabinoid System Dysregulation from Acetaminophen Use May Lead to Autism Spectrum Disorder: Could Cannabinoid Treatment Be Efficacious?
molecules Review Endocannabinoid System Dysregulation from Acetaminophen Use May Lead to Autism Spectrum Disorder: Could Cannabinoid Treatment Be Efficacious? Stephen Schultz 1, Georgianna G. Gould 1, Nicola Antonucci 2, Anna Lisa Brigida 3 and Dario Siniscalco 4,* 1 Department of Cellular and Integrative Physiology, Center for Biomedical Neuroscience, University of Texas (UT) Health Science Center San Antonio, San Antonio, TX 78229, USA; [email protected] (S.S.); [email protected] (G.G.G.) 2 Biomedical Centre for Autism Research and Therapy, 70126 Bari, Italy; [email protected] 3 Department of Precision Medicine, University of Campania, 80138 Naples, Italy; [email protected] 4 Department of Experimental Medicine, University of Campania, 80138 Naples, Italy * Correspondence: [email protected] Abstract: Persistent deficits in social communication and interaction, and restricted, repetitive pat- terns of behavior, interests or activities, are the core items characterizing autism spectrum disorder (ASD). Strong inflammation states have been reported to be associated with ASD. The endocannabi- noid system (ECS) may be involved in ASD pathophysiology. This complex network of lipid signal- ing pathways comprises arachidonic acid and 2-arachidonoyl glycerol-derived compounds, their G-protein-coupled receptors (cannabinoid receptors CB1 and CB2) and the associated enzymes. Alter- Citation: Schultz, S.; Gould, G.G.; ations of the ECS have been reported in both the brain and the immune system of ASD subjects. ASD Antonucci, N.; Brigida, A.L.; Siniscalco, D. Endocannabinoid children show low EC tone as indicated by low blood levels of endocannabinoids. Acetaminophen System Dysregulation from use has been reported to be associated with an increased risk of ASD. -

The Cannabinoid WIN 55,212-2 Prevents Neuroendocrine Differentiation of Lncap Prostate Cancer Cells
OPEN Prostate Cancer and Prostatic Diseases (2016) 19, 248–257 www.nature.com/pcan ORIGINAL ARTICLE The cannabinoid WIN 55,212-2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells C Morell1, A Bort1, D Vara2, A Ramos-Torres1, N Rodríguez-Henche1 and I Díaz-Laviada1 BACKGROUND: Neuroendocrine (NE) differentiation represents a common feature of prostate cancer and is associated with accelerated disease progression and poor clinical outcome. Nowadays, there is no treatment for this aggressive form of prostate cancer. The aim of this study was to determine the influence of the cannabinoid WIN 55,212-2 (WIN, a non-selective cannabinoid CB1 and CB2 receptor agonist) on the NE differentiation of prostate cancer cells. METHODS: NE differentiation of prostate cancer LNCaP cells was induced by serum deprivation or by incubation with interleukin-6, for 6 days. Levels of NE markers and signaling proteins were determined by western blotting. Levels of cannabinoid receptors were determined by quantitative PCR. The involvement of signaling cascades was investigated by pharmacological inhibition and small interfering RNA. RESULTS: The differentiated LNCaP cells exhibited neurite outgrowth, and increased the expression of the typical NE markers neuron-specific enolase and βIII tubulin (βIII Tub). Treatment with 3 μM WIN inhibited NK differentiation of LNCaP cells. The cannabinoid WIN downregulated the PI3K/Akt/mTOR signaling pathway, resulting in NE differentiation inhibition. In addition, an activation of AMP-activated protein kinase (AMPK) was observed in WIN-treated cells, which correlated with a decrease in the NE markers expression. Our results also show that during NE differentiation the expression of cannabinoid receptors CB1 and CB2 dramatically decreases. -
![Modulation of Neuropathic and Inflammatory Pain by the Endocannabinoid Transport Inhibitor AM404 [N-(4-Hydroxyphenyl)-Eicosa-5,8,11,14-Tetraenamide]](https://docslib.b-cdn.net/cover/4659/modulation-of-neuropathic-and-inflammatory-pain-by-the-endocannabinoid-transport-inhibitor-am404-n-4-hydroxyphenyl-eicosa-5-8-11-14-tetraenamide-1214659.webp)
Modulation of Neuropathic and Inflammatory Pain by the Endocannabinoid Transport Inhibitor AM404 [N-(4-Hydroxyphenyl)-Eicosa-5,8,11,14-Tetraenamide]
0022-3565/06/3173-1365–1371$20.00 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS Vol. 317, No. 3 Copyright © 2006 by The American Society for Pharmacology and Experimental Therapeutics 100792/3113920 JPET 317:1365–1371, 2006 Printed in U.S.A. Modulation of Neuropathic and Inflammatory Pain by the Endocannabinoid Transport Inhibitor AM404 [N-(4-Hydroxyphenyl)-eicosa-5,8,11,14-tetraenamide] G. La Rana,1 R. Russo,1 P. Campolongo, M. Bortolato, R. A. Mangieri, V. Cuomo, A. Iacono, G. Mattace Raso, R. Meli, D. Piomelli, and A. Calignano Department of Experimental Pharmacology, University of Naples, Naples, Italy (G.L.R., R.R., A.I., G.M.R., R.M., A.C.); Department of Human Physiology and Pharmacology, University of Rome “La Sapienza,” Rome, Italy (P.C., V.C.); and Department of Pharmacology and Center for Drug Discovery, University of California, Irvine, California (M.B., R.A.M., D.P.) Received December 29, 2005; accepted February 28, 2006 ABSTRACT The endocannabinoid system may serve important functions in (30 mg/kg i.p.). Comparable effects were observed with the central and peripheral regulation of pain. In the present UCM707 [N-(3-furylmethyl)-eicosa-5,8,11,14-tetraenamide], study, we investigated the effects of the endocannabinoid another anandamide transport inhibitor. In both the chronic transport inhibitor AM404 [N-(4-hydroxyphenyl)-eicosa- constriction injury and complete Freund’s adjuvant model, daily 5,8,11,14-tetraenamide] on rodent models of acute and persis- treatment with AM404 (1–10 mg/kg s.c.) for 14 days produced tent nociception (intraplantar formalin injection in the mouse), a dose-dependent reduction in nocifensive responses to ther- neuropathic pain (sciatic nerve ligation in the rat), and inflam- mal and mechanical stimuli, which was prevented by a single matory pain (complete Freund’s adjuvant injection in the rat). -

The Role of the Endocannabinoid System
International Journal of Molecular Sciences Review The Impact of Early Life Exposure to Cannabis: The Role of the Endocannabinoid System Annia A. Martínez-Peña 1,2,†, Genevieve A. Perono 1,2,† , Sarah Alexis Gritis 2, Reeti Sharma 3, Shamini Selvakumar 3, O’Llenecia S. Walker 2,3, Harmeet Gurm 2,3, Alison C. Holloway 1,2 and Sandeep Raha 2,3,* 1 Graduate Program in Medical Sciences, McMaster University, Hamilton, ON L8S 4K1, Canada; [email protected] (A.A.M.-P.); [email protected] (G.A.P.); [email protected] (A.C.H.) 2 Department of Obstetrics and Gynecology, McMaster University, Hamilton, ON L8S 4K1, Canada; [email protected] (S.A.G.); [email protected] (O.S.W.); [email protected] (H.G.) 3 Department of Pediatrics, McMaster University, Hamilton, ON L8S 4K1, Canada; [email protected] (R.S.); [email protected] (S.S.) * Correspondence: [email protected]; Tel.: +1-905-521-2100 (ext. 76213) † These authors contributed equally to the preparation of this manuscript. Abstract: Cannabis use during pregnancy has continued to rise, particularly in developed countries, as a result of the trend towards legalization and lack of consistent, evidence-based knowledge on the matter. While there is conflicting data regarding whether cannabis use during pregnancy leads to adverse outcomes such as stillbirth, preterm birth, low birthweight, or increased admission to neonatal intensive care units, investigations into long-term effects on the offspring’s health are limited. Historically, studies have focused on the neurobehavioral effects of prenatal cannabis exposure on Citation: Martínez-Peña, A.A.; the offspring. -

Acetaminophen Relieves Inflammatory Pain Through CB1 Cannabinoid Receptors in the Rostral Ventromedial Medulla
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Systems/Circuits Acetaminophen Relieves Inflammatory Pain Through CB1 Cannabinoid Receptors in the Rostral Ventromedial Medulla Pascal P. Klinger-Gratz1, William T. Ralvenius1, Elena Neumann1, Ako Kato2, Rita Nyilas2, Zsolt Lele2, István Katona2 and Hanns Ulrich Zeilhofer1,3 1Institute of Pharmacology and Toxicology, University of Zurich, Winterthurerstrasse 190, CH-8057, Switzerland 2Institute of Experimental Medicine, Hungarian Academy of Sciences, 43 Szigony Street, H-1083 Budapest, Hungary 3Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology (ETH) Zurich, Vladimir-Prelog-Weg 1-5/10, CH-8093 Zürich, Switzerland DOI: 10.1523/JNEUROSCI.1945-17.2017 Received: 9 July 2017 Revised: 27 October 2017 Accepted: 14 November 2017 Published: 22 November 2017 Author contributions: P.P.K.-G., W.T.R., E.N., A.K., R.N., Z.L., and I.K. performed research; P.P.K.-G., W.T.R., E.N., A.K., R.N., Z.L., I.K., and H.U.Z. analyzed data; W.T.R., I.K., and H.U.Z. designed research; I.K. and H.U.Z. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. The authors thank Drs. Beat Lutz and Giovanni Marsicano for providing CB1fl/fl mice, Dr. Masahiko Watanabe for the CB1 receptor antibody, Sébastien Druart, Andreas Pospischil, Roseline Weilenmann for the analyses of biomarkers of liver damage, and Isabelle Kellenberger, Balázs Pintér, Erika Tischler and Louis Scheurer for technical assistance. The work was partially supported by a grant from Federal Government of Switzerland through the Swiss Contribution (SH7/2/18) to IK and HUZ and by the Hungarian Academy of Sciences Momentum Program LP-54/2013 (to IK). -

Cannabinoid Ligands Targeting TRP Channels By
Cannabinoid ligands targeting TRP channels By: Chanté Muller, Paula Morales, and Patricia H. Reggio Muller, C., Morales, P., & Reggio, P.H. (2019). Cannabinoid ligands targeting TRP channels. Frontiers in Molecular Neuroscience, 11, 497. https://doi.org/10.3389/fnmol.2018.00487 © 2019 Muller, Morales and Reggio. Published under a Creative Commons Attribution International License (CC BY 4.0); https://creativecommons.org/licenses/by/4.0/. Abstract: Transient receptor potential (TRP) channels are a group of membrane proteins involved in the transduction of a plethora of chemical and physical stimuli. These channels modulate ion entry, mediating a variety of neural signaling processes implicated in the sensation of temperature, pressure, and pH, as well as smell, taste, vision, and pain perception. Many diseases involve TRP channel dysfunction, including neuropathic pain, inflammation, and respiratory disorders. In the pursuit of new treatments for these disorders, it was discovered that cannabinoids can modulate a certain subset of TRP channels. The TRP vanilloid (TRPV), TRP ankyrin (TRPA), and TRP melastatin (TRPM) subfamilies were all found to contain channels that can be modulated by several endogenous, phytogenic, and synthetic cannabinoids. To date, six TRP channels from the three subfamilies mentioned above have been reported to mediate cannabinoid activity: TRPV1, TRPV2, TRPV3, TRPV4, TRPA1, and TRPM8. The increasing data regarding cannabinoid interactions with these receptors has prompted some researchers to consider these TRP channels to be “ionotropic cannabinoid receptors.” Although CB1 and CB2 are considered to be the canonical cannabinoid receptors, there is significant overlap between cannabinoids and ligands of TRP receptors. The first endogenous agonist of TRPV1 to be discovered was the endocannabinoid, anandamide (AEA). -

Neuropsychiatric Implications of Transient Receptor Potential Vanilloid (TRPV) Channels in the Reward System T
Neurochemistry International 131 (2019) 104545 Contents lists available at ScienceDirect Neurochemistry International journal homepage: www.elsevier.com/locate/neuint Neuropsychiatric implications of transient receptor potential vanilloid (TRPV) channels in the reward system T ∗∗ ∗ Raghunath Singha, Yashika Bansala, Ishwar Parharb, Anurag Kuhada, , Tomoko Sogab, a Pharmacology Division, University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, 160014, India b Brain Research Institute Monash Sunway (BRIMS), Jeffrey Cheah School of Medicine and Health Science, Monash University Malaysia, Bandar Sunway, 47500, Malaysia ARTICLE INFO ABSTRACT Keywords: Neuropsychiatric disorders (NPDs) exert a devastating impact on an individual's personal and social well-being, TRPV channels encompassing various conditions and brain anomalies that influence affect, cognition, and behavior. Because the Addiction pathophysiology of NPDs is multifactorial, the precise mechanisms underlying the development of such disorders Food reward remain unclear, representing a unique challenge in current neuropsychopharmacotherapy. Transient receptor Opioids potential vanilloid (TRPV) type channels are a family of ligand-gated ion channels that mainly include sensory Alcohol receptors that respond to thermal, mechanical and chemical stimuli. TRPV channels are abundantly present in Cannabinoids dopaminergic neurons, thus playing a pivotal role in the modulation of the reward system and in pathophy- siology of diseases such as stress, anxiety, depression, schizophrenia, neurodegenerative disorders and substance abuse/addiction. Recent evidence has highlighted TRPV channels as potential targets for understanding mod- ulation of the reward system and various forms of addiction (opioids, cocaine, amphetamines, alcohol, nicotine, cannabis). In this review, we discuss the distribution, physiological roles, ligands and therapeutic importance of TRPV channels with regard to NPDs and addiction biology. -
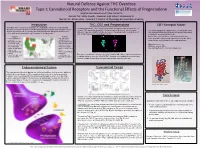
Introduction Endocannabinoid System Cannabinoid Tetrad THC, CB1 And
Natural Defense Against THC Overdose Type-1 Cannabinoid Receptors and the Functional Effects of Pregnenolone Brigitte Rios Llamosa and Alexis Camacho Advisor: Mr. Justin Spaeth, Messmer High School, Milwaukee WI Mentor: Dr. Aaron Miller - Assistant Professor of Physiology at Concordia University Introduction THC, CB1 and Pregnenolone CB1 Receptor Model Cannabis, often referred to as marijuana, is a drug produced from the Cannabis plant. Tetrahydrocannabinol (THC), the active ingredient in marijuana, also activates the CB1 Recently, marijuana has become an often debated topic as people work to legalize its use receptor. THC and similar drugs have therapeutic potential in the treatment of pain, Our model highlights the amino acids E133 and R409, which for both recreational (as in Colorado) and medical purposes. Marijuana is able to help Alzheimer’s disease, anxiety, arthritis, and cancer. A downside to the medicinal use of form hydrogen bonds with pregnenolone and are required for relieve pain, but it can also lower performance in everyday tasks. THC is that it also induces psychotropic effects. its binding to the allosteric site of CB1. Negatives Positives Both of these amino acids are colored in cpk color and • Poor coordination of • Can help control connected with a strut to our pregnenolone molecule. Other movement epileptic seizures areas that are highlighted are color-coded as follows: • Afterwards, users feel • May decrease anxiety tired or depressed • Can slow progression Alpha helixes are red. • Increases heartbeat and of diseases such as risk of heart attack Alzheimer’s disease Struts are colored white. • Inability to understand • Has been used to treat Non-motif portions are colored cornflower blue. -

Cannabis and Endocannabinoid System
ISSN: 2455-3484 DOI: https://dx.doi.org/10.17352/jamts MEDICAL GROUP Received: 28 December, 2020 Mini Review Accepted: 18 January, 2021 Published: 19 January, 2021 *Corresponding author: Somchai Amornyotin, As- Cannabis and sociate Professor, Department of Anesthesiology and Siriraj GI Endoscopy Center, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand, Endocannabinoid System Tel: 66-2-419-7990; Fax: 662-4113256; E-mail: Somchai Amornyotin* ORCID: https://orcid.org/0000-0002-4345-5836 Department of Anesthesiology and Siriraj GI Endoscopy Center, Faculty of Medicine Siriraj Hospital, Keywords: Cannabis; Endocannabinoid system; Mahidol University, Bangkok, Thailand Cannabinoid; Marijuana https://www.peertechz.com Abstract The endocannabinoid system is involved in various physiological pathways in the human body. A large number of endogenous cannabinoids have been acknowledged, and cannabinoid receptor type 1 (CB-1) and cannabinoid receptor type 2 (CB-2) have been categorized. The activation of endocannabinoid system could regulate the activity in several neural pathways. The complex functions of this system have generated multiple new targets for pharmacotherapies. Several research studies have concentrated on these issues. However, these efforts have been generally unsuccessful. Although cannabinoids have therapeutic potential, their psychoactive properties have largely limited their usage in clinical practice. In this review, the author briefly summarized the knowledge of cannabis and the endocannabinoid system. Introduction The human body has a cannabis chemical producing factory called the endocannabinoid system. All vertebrate In 1964, Raphael Mechoulam and colleagues at Hebrew species, including humans, have an endocannabinoid system. University in Jerusalem isolated Tetrahydrocannabinol The endocannabinoid system is one of the great unknowns of (THC) and found it to be the main psychoactive compound in the biology. -

The Endocannabinoid System and Invertebrate Neurodevelopment and Regeneration
International Journal of Molecular Sciences Review The Endocannabinoid System and Invertebrate Neurodevelopment and Regeneration Tristyn L. Clarke 1, Rachael L. Johnson 1 , Jonathan J. Simone 1,2,3 and Robert L. Carlone 1,2,* 1 Department of Biological Sciences, Brock University, 1812 Sir Isaac brock Way, St. Catharines, ON L2S 3A1, Canada; [email protected] (T.L.C.); [email protected] (R.L.J.); [email protected] (J.J.S.) 2 Centre for Neuroscience, Brock University, 1812 Sir Isaac brock Way, St. Catharines, ON L2S 3A1, Canada 3 eCB Consulting Inc., P.O. Box 652, 3 Cameron St. W., Cannington, ON L2S 3A1, Canada * Correspondence: [email protected] Abstract: Cannabis has long been used for its medicinal and psychoactive properties. With the rela- tively new adoption of formal medicinal cannabis regulations worldwide, the study of cannabinoids, both endogenous and exogenous, has similarly flourished in more recent decades. In particular, research investigating the role of cannabinoids in regeneration and neurodevelopment has yielded promising results in vertebrate models. However, regeneration-competent vertebrates are few, whereas a myriad of invertebrate species have been established as superb models for regeneration. As such, this review aims to provide a comprehensive summary of the endocannabinoid system, with a focus on current advances in the area of endocannabinoid system contributions to invertebrate neurodevelopment and regeneration. Keywords: AEA; 2-AG; CB1; CB2; endocannabinoid; regeneration; neurodevelopment; invertebrate Citation: Clarke, T.L.; Johnson, R.L.; Simone, J.J.; Carlone, R.L. The Endocannabinoid System and Invertebrate Neurodevelopment and 1. Historical Introduction to Cannabis and Endocannabinoids Regeneration. Int. J. Mol. Sci.