Clinical Trial Protocol Iranian Registry of Clinical Trials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Faisalabad Chamber of Commerce & Indusrty
THE FAISALABAD CHAMBER OF COMMERCE & INDUSRTY FINAL ASSOCIATE VOTERS FOR THE YEAR 2016-17 Page 1 of 142 1. 2104577-3610 7. 2106048-4897 13. 2103964-3062 3-A TEXTILES A & H CORPORATION A.A ENTERPRISES P-1 SECOND FLOOR SARA TOWER CENTRE OFFICE # 4, 2ND FLOOR ABUTURAB P-27 GALI NO 7 MOHALLAH QASIM MAIN SUSAN ROAD MADINA BUILDING, AMINPUR BAZAR FAISALABAD ABAD,JHANG ROAD, FAISALABAD TOWN,FAISALABAD NTN : 2681221-5 NTN : 2259549-0 NTN : 1324438-8 STN : STN : STN : 08-90-9999-882-82 TEL : 041-8559844,5506962 TEL : 041-2551678 TEL : 041-8723388 CELL: 0300-9656930 CELL: 0300-9652354,0321-7220920 CELL: 0300-8668801 EMAIL :[email protected], rana_248@hotmai EMAIL :[email protected] EMAIL :[email protected] REP : RANA MUNAWAR HUSSAIN REP : HAMID BASHIR REP : AIJAZ AHMAD NIC : 33100-0147581-1 NIC : 33100-4747059-7 NIC : 33100-5628937-9 2. 2106191-5024 8. 2300939-682 14. 2102967-2222 381 INTERNATIONAL A & H ENTERPRISES A.A ENTERPRISES 1ST FLOOR UNION TRADER OPP. SHELL CHEEMA MARKET RAILWAY ROAD SHOP NO 4 AL HAKEEM CENTRE CHTTREE PUMP NEAR GHOUSHALA DIJKOT ROAD FAISALABAD WALA CHOWK JINNAH COLONY FAISALABAD NTN : 2164711-9 FAISALABAD NTN : 2736536-7 STN : 08-01-5900-008-46 NTN : 2316510-3 STN : TEL : 041-2643933 STN : TEL : 041-2626381 CELL: 0300-8666818 TEL : 041-8739180 CELL: 0344-4444381 EMAIL :[email protected] CELL: 0300-8656607 EMAIL :[email protected] REP : MUHAMMAD ASIM EMAIL :[email protected] REP : JAWAD ALI NIC : 33100-1808192-7 REP : ATIF IDREES NIC : 33100-6169762-5 NIC : 33104-2111449-9 3. 2105589-4504 9. -
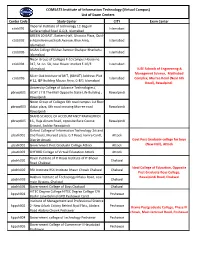
COMSATS Institute of Information Technology (Virtual Campus) List of Exam Centers
COMSATS Institute of Information Technology (Virtual Campus) List of Exam Centers Center Code Study Center CITY Exam Center Imperial Institute of technology 12-Begum ctisb001 Islamabad Sarfaraz Iqbal Road G-6/4, Islamabad GREEN 20-EAST, Eastern Half, Ghousia Plaza, Quid- ctisb002 e-AzamAvenue/Jinah Avanue, Blue Area, Islamabad Islamabad ASIAN College Iftikhar Avenue Shahpur Bharkahu ctisb003 Islamabad Islamabad Nicon Group of Colleges F-10 campus House no. ctisb004 317, St. no. 58, near flower market F-10/3 Islamabad Islamabad IUSE Schools of Engineering & Managment Science, Malikabad Silver Oak Institute of MIT, (SIMSIT) Address: Plot ctisb006 Islamabad Complex, Murree Road (Near 6th # 12, IEP Building Mauve Area, G-8/1 Islamabad Road), Rawalpindi University College of Advance Technologies ( pbrwp001 UCAT ) 7 B The Mall Opposite State Life Building , Rawalpindi Rawalpindi Nicon Group of Colleges 6th road campus 1st floor pbrwp003 dubai plaza, 6th road crossing Murree road Rawalpindi Rawalpindi SKANS SCHOOL OF ACCOUNTANCY RAWALPINDI pbrwp005 16 , Raja Akram Road, opposite Race Course Rawalpindi Ground, Saddar Rawalpindi Oxford College of Information Technology 1st and pbatk001 2nd floors, Mureed plaza, G.T Road, kamra Cantt, Attock District Attock Govt Post Graduate college for boys pbatk002 Government Post Graduate College Attock Attock (New Hall), Attock pbatk003 OXFORD College of Virtual Education Attock Attock Royal Institute of IT Royal Institute of IT Bhoun pbchk001 Chakwal Road Chakwal Ideal College of Education, Opposite pbchk002 -

List of Branches Authorized for Overnight Clearing (Annexure - II) Branch Sr
List of Branches Authorized for Overnight Clearing (Annexure - II) Branch Sr. # Branch Name City Name Branch Address Code Show Room No. 1, Business & Finance Centre, Plot No. 7/3, Sheet No. S.R. 1, Serai 1 0001 Karachi Main Branch Karachi Quarters, I.I. Chundrigar Road, Karachi 2 0002 Jodia Bazar Karachi Karachi Jodia Bazar, Waqar Centre, Rambharti Street, Karachi 3 0003 Zaibunnisa Street Karachi Karachi Zaibunnisa Street, Near Singer Show Room, Karachi 4 0004 Saddar Karachi Karachi Near English Boot House, Main Zaib un Nisa Street, Saddar, Karachi 5 0005 S.I.T.E. Karachi Karachi Shop No. 48-50, SITE Area, Karachi 6 0006 Timber Market Karachi Karachi Timber Market, Siddique Wahab Road, Old Haji Camp, Karachi 7 0007 New Challi Karachi Karachi Rehmani Chamber, New Challi, Altaf Hussain Road, Karachi 8 0008 Plaza Quarters Karachi Karachi 1-Rehman Court, Greigh Street, Plaza Quarters, Karachi 9 0009 New Naham Road Karachi Karachi B.R. 641, New Naham Road, Karachi 10 0010 Pakistan Chowk Karachi Karachi Pakistan Chowk, Dr. Ziauddin Ahmed Road, Karachi 11 0011 Mithadar Karachi Karachi Sarafa Bazar, Mithadar, Karachi Shop No. G-3, Ground Floor, Plot No. RB-3/1-CIII-A-18, Shiveram Bhatia Building, 12 0013 Burns Road Karachi Karachi Opposite Fresco Chowk, Rambagh Quarters, Karachi 13 0014 Tariq Road Karachi Karachi 124-P, Block-2, P.E.C.H.S. Tariq Road, Karachi 14 0015 North Napier Road Karachi Karachi 34-C, Kassam Chamber's, North Napier Road, Karachi 15 0016 Eid Gah Karachi Karachi Eid Gah, Opp. Khaliq Dina Hall, M.A. -

Urban Development
URBAN DEVELOPMENT Urban Development Sector covers development projects sponsored by WASAs and Development Authorities (DAs) of large cities (Lahore, Faisalabad, Rawalpindi, Gujranwala & Multan) and of Bahawalpur Development Authority (BDA) and Fort Monroe Development Authority (FDMA). VISION Provision of adequate and efficient urban services in cities and harnessing their potential for making them engines of economic growth. POLICY Promoting integrated land use and infrastructure development, urban renewal and regeneration for improving accessibility, mitigating climate change risks and building inclusive urban communities. OBJECTIVES Supply of potable drinking water and its efficient use Provision of effective and efficient sewerage and drainage system Environment friendly disposal of sewage Safe and efficient roads infrastructure Provision of solid waste management services Strategic planning for growth of cities on scientific lines STRATEGY A central principle of Urban Strategy is that “density” and “agglomeration” are central to economic development, higher productivity, social equity and human development. To make Punjab competitive for investment and development, cities are going to play a vital role, because they can benefit from a large and skilled labor force, economies of urban scale, economies of agglomeration (i.e. efficiency resulting from clustering of firms in a given industry or related industries), and the resulting demand for goods and services. 429 Further, rural-urban migration and urbanization can only lead to higher income if manufacturing and services grow fast enough to absorb the supply of labor. Pakistan will have to invest in many cities at the same time to ensure a more geographically balanced rate of urbanization and the creation of a system of cities an efficient network of urban centers whose manufacturing and services industry are connected. -

HCAR CDC LIST AS on MARCH 21, 2018 Sr No Member Folio Parid
HCAR CDC LIST AS ON MARCH 21, 2018 Sr No Member Folio ParID CDC A/C # SHARE HOLDER'S NAME ADDRESS 1 CDC-1 00208 30 ALFA ADHI SECURITIES (PVT) LTD. SUITE NO. 303, 3RD FLOOR, MUHAMMAD BIN QASIM ROAD, I.I. CHUNDRIGAR ROAD KARACHI 2 CDC-2 00208 3075 Rafiq III-F, 13/7, NAZIMABAD NO. 3, 74600 KARACHI 3 CDC-3 00208 3729 Omar Farooq HOUSE NO B-144, BLOCK-3, GULISTAN-E-JOUHAR KARACHI 4 CDC-4 00208 7753 AFSHAN SHAKIL A-82, S.B.C.H.S. BLOCK 12, GULISTAN-E-JAUHAR KARACHI 5 CDC-5 00208 8827 SARWAR DIN L-361, SHEERIN JINNAH COLONY , CLIFTON KARACHI 6 CDC-6 00208 11805 SHUNIL KUMAR OFFICE # 105,HUSSAIN TRADE CENTER, ALTAF HUSSAIN ROAD, NEW CHALI KARACHI 7 CDC-7 00208 12126 ABDUL SAMAD D-9, DAWOOD COLONY, SHARFABAD, NEAR T.V. STATION. KARACHI 8 CDC-8 00208 18206 Syeda Sharmeen Flat # 506, Ana Classic Apartment, Ghulam Hussain Qasim Road, Garden West KARACHI 9 CDC-9 00208 18305 Saima Asif FLAT NO: 306, MOTIWALA APARTMENT, PLOT NO: BR 5/16, MULJI STREET, KHARADHAR KARACHI 10 CDC-10 00208 18511 Farida FLAT NO: 306, MOTIWALA APARTMENT, PLOT NO: BR 5/16, MULJI STREET, KHARADHAR, KARACHI 11 CDC-11 00208 19626 MANOHAR LAL MANOHAR EYE CLINIC JACOBABAD 12 CDC-12 00208 21945 ADNAN FLAT NO 101, PLOT NO 329, SIDRA APPARTMENT, GARDEN EAST, BRITTO ROAD, KARACHI 13 CDC-13 00208 25672 TAYYAB IQBAL FLAT NO. 301, QASR-E-GUL, NEAR T.V. STATION, JAMAL-UD-DIN AFGHANI ROAD, SHARFABAD KARACHI 14 CDC-14 00208 26357 MUHAMMAD SALEEM KHAN G 26\9,LIAQUAT SQUARE, MALIR COLONY, KARACHI 15 CDC-15 00208 27165 SABEEN IRFAN 401,HEAVEN PRIDE,BLOCK-D, FATIMA JINNAH COLONY,JAMSHED ROAD#2, KARACHI 16 CDC-16 00208 27967 SANA UL HAQ 3-F-18-4, NAZIMABAD KARACHI 17 CDC-17 00208 29112 KHALID FAREED SONIA SQUARE, FLAT # 05, STADIUM ROAD, CHANDNI CHOWK, KARACHI 5 KARACHI 18 CDC-18 00208 29369 MOHAMMAD ZEESHAN AHMED FLAT NO A-401, 4TH FLOOR, M.L GARDEN PLOT NO, 690, JAMSHED QUARTERS, KARACHI 19 CDC-19 00208 30151 SANIYA MUHAMMAD RIZWAN HOUSE # 4, PLOT # 3, STREET# 02, MUSLIMABAD, NEAR DAWOOD ENGG. -

Faisalabad Chamber of Small Traders & Small Industry (Fcsti)
FAISALABAD CHAMBER OF SMALL TRADERS & SMALL INDUSTRY (FCSTI) VOTTER LIST FOR ELECTION 2019-20 Sr. Membership No. Company Name Business Address NTN of the company Name of Authorized representative CNIC NO No. 1 FCSTI-00001 Mian CNG Filling Station Chak No. 226 R.B, Main Satiana Road, Faisalabad 2676988-3 Zafar Iqbal 33100-4433087-5 2 FCSTI-00002 M. Amin & Company P-125, ST. No. 3, Gulistan Market, Railway Road, Faisalabad 1426981-3 Muhammad Amin 33100-7922660-9 3 FCSTI-00003 M. Ashraf Weaving Factory 545-A G. M. Abad, Faisalabad 0792700-2 Malik Muhammad Ashraf 33100-2090767-7 4 FCSTI-00006 Pacific Trading Co Shop 103-104 ST No. 3 Gulistan Market,Railway Road, FSD 0950024-3 Abdul Hameed Dar 33100-0785147-9 5 FCSTI-00007 German Garments H. 206-207 Maqbool Town No. 1, Chak No. 100 JB, FSD. 1316232-2 FARHAJ MUSHTAQ 33100-0988594-5 6 FCSTI-00008 Fresh Flour Mills Bazar No 1, Raza Abad, Faisalabad 3165269-7 Muhammad Shafiq Anjum 33100-2713450-9 7 FCSTI-00009 Aftab Weavings Pensara Road, Chak No. 363 J.B, Tehsil Gojra 1244522-3 Haji Muhammad Tufail 33301-1320896-5 8 FCSTI-00010 Tlha Asif Weaving Factory P-64, St. # 3, Talha Asif Plaza, 1st Floor, Karkhana Bazar, Faisalabad 2907975-6 Muhammad Asif Rafiq 33100-8662605-1 9 FCSTI-00011 Total IT Solutions P-114, Street No.3, Gulistan Market, Railway Road, Faisalabad 2030912-7 Raza Hussnain 38403-2239053-3 10 FCSTI-00012 Umar Embroidery Opp Khayaban-e-Manzoor Near Al Shifa International Hospital, Faisalabad 2445726-4 Umar Aftab 33301-7299360-9 11 FCSTI-00013 A.S Textile 1.5 KM, Jaranwala Road, Kharrad Wala Road, Faisalabad 3797648-6 Hamid Ali 33104-1264266-7 12 FCSTI-00017 Computer House 126-B Basement Rex City, Satiana Road, Faisalabad 2439501-3 Muhammad Sarfraz 33100-9722503-1 13 FCSTI-00018 Naseem Packages (Pvt.)Ltd. -

Appendices Appendix-I Number of Reporting Scheduled Banks and Their Branches (1980-2015)
Appendices Appendix-I Number of Reporting Scheduled Banks and Their Branches (1980-2015) As on Pakistani Banks Foreign Banks Total 30th June No. of Banks No. of Branches No. of Banks No. of Branches No. of Banks No. of Branches 1980 9 6,723 21 56 30 6,779 1981 9 7,103 21 56 30 7,159 1982 9 7,244 22 57 31 7,301 1983 9 7,195 23 59 32 7,254 1984 9 7,051 23 59 32 7,110 1985 9 6,980 23 59 32 7,039 1986 9 6,955 22 60 31 7,015 1987 9 7,023 23 63 32 7,086 1988 9 7,141 28 65 37 7,206 1989 10 7,188 25 66 34 7,254 1990 10 7,337 26 67 36 7,404 1991 10 7,480 26 69 36 7,549 1992 20 7,538 28 71 46 7,609 1993 20 7,634 27 71 47 7,705 1994 21 7,663 26 72 47 7,735 1995 25 8,200 25 73 50 8,273 1996 25 8,387 28 82 53 8,469 1997 25 8,446 27 84 52 8,530 1998 25 7,921 27 91 52 8,012 1999 25 7,841 27 94 52 7,935 2000 25 7,755 26 92 51 7,847 2001 24 7,165 25 88 49 7,253 2002 25 6,878 24 88 49 6,966 2003 24 6,834 22 82 46 6,916 2004 28 6,803 17 79 45 6,882 2005 28 7,014 17 91 45 7,105 2006 30 7,296 17 125 47 7,421 2007 34 7,693 13 64 47 7,757 2008 33 8,277 12 69 45 8,346 2009 33 8,686 13 97 46 8,783 2010 33 9,007 13 89 46 9,096 2011 32 9,341 12 58 44 9,399 2012 31 9,792 13 55 44 9,847 2013 31 10,332 7 29 38 10,361 2014 31 10,957 7 27 38 10,984 2015 30 11,705 5 11 35 11,716 120 Appendix-II Reporting Scheduled Banks &Their Branches by Group (June 30th ,2015) No. -

Urban Development
URBAN DEVELOPMENT Urban Development Sector covers development projects sponsored by WASAs and Development Authorities (DAs) of large cities (Lahore, Faisalabad, Rawalpindi, Gujranwala & Multan) and of Bahawalpur Development Authority (BDA) and Fort Monroe Development Authority (FDMA). VISION Provision of adequate and efficient urban services in cities and harnessing their potential for making them engines of economic growth. POLICY Promoting integrated land use and infrastructure development, urban renewal and regeneration for improving accessibility, mitigating climate change risks and building inclusive urban communities. OBJECTIVES · Supply of potable drinking water and its efficient use · Provision of effective and efficient sewerage and drainage system · Environment friendly disposal of sewage · Safe and efficient roads infrastructure · Provision of solid waste management services · Strategic planning for growth of cities on scientific lines STRATEGY · A central principle of Urban Strategy is that “density” and “agglomeration” are central to economic development, higher productivity, social equity and human development. To make Punjab competitive for investment and development, cities are going to play a vital role, because they can benefit from a large and skilled labor force, economies of urban scale, economies of agglomeration (i.e. efficiency resulting from clustering of firms in a given industry or related industries), and the resulting demand for goods and services. 429 · Further, rural-urban migration and urbanization can only lead to higher income if manufacturing and services grow fast enough to absorb the supply of labor. Pakistan will have to invest in many cities at the same time to ensure a more geographically balanced rate of urbanization and the creation of a system of cities an efficient network of urban centers whose manufacturing and services industry are connected. -
Date : 26/07/21 Detail of Sub-Division/Feeder Wise Distribution Losses Company Fesco : for the Period from 04/2021 to 06
DATE : 26/07/21 DETAIL OF SUB-DIVISION/FEEDER WISE DISTRIBUTION LOSSES COMPANY FESCO : FOR THE PERIOD FROM 04/2021 TO 06/2021 ALL FIGURE IN MILLION Sub Division/ UNITS UNITS DIVISION/CIRCLE FEEDER %AGE LOSSES BILLING Collection %AGE RECEIVED SOLD NAME 13121 F/ABAD CITY 47510 MODEL TOWN 3.31 2.95 10.77% 1.54 67.72 2.41 49.7 75.24% 61814 JHANG BAZAR 2.18 1.7 21.91% 0.08 46.84 0.1 45.72 97.66% 61824 IMAM BARGAH ROAD 2.78 2.39 13.95% 6.44 50.35 5.59 45.11 89.27% 117713 CIRCULAR ROAD 1.67 1.35 19.14% 1.5 38.21 2.46 35.37 95.26% 117715 MONTGOMERY 1.39 1.22 11.73% 0.46 32.39 0.57 31 96.10% SUB-DIVISION TOTAL 11.33 9.62 15.05% 10.03 235.51 11.13 207.21 88.92% 13122 F/ABAD CIVIL LI 43106 D.H.Q. 1.14 1.15 -0.66% 11.24 20.49 11.75 14.15 81.64% 43121 CIVIL LINE 2.85 2.63 7.75% 11.49 57 13.41 45.63 86.21% 43130 CARDIOLOGY 0.76 1.09 -42.72% 27.67 0 44.54 0 160.95% 47507 STATE BANK 1 3.76 3.42 9.18% 1.15 76.52 2.5 52.95 71.39% 47517 AGRI-UNIVERSITY 2.09 1.95 6.70% 34.61 12.35 28.03 8.84 78.50% 47518 CITY 2.48 1.98 20.17% 2.44 50.8 1.91 44.33 86.85% 47520 SAREENA 0.48 0.48 -0.49% 0 14.45 0 12.81 88.67% 47521 STATE LIFE 0.18 0.21 -16.19% 0 7 0 3.83 54.72% 47525 IQBAL STADIUM 0 0 0.00% 0 0 0 0 0.00% 47527 EID GAH ROAD 3.42 3.13 8.47% 23.01 65.53 35.96 52.54 99.95% CARDIOLOGY-2 (BUNDLE 47534 043130) 0.27 0 100.00% 0 0 0 0 0.00% 61809 RAILWAY ROAD 0 0.09 0.00% 0.55 2.41 0.79 2.04 95.36% 61810 KATCHARY ROAD 3.29 2.4 26.84% 12.7 55.69 17.16 53.32 103.06% 61819 GOAL KARYANA 1.47 1.06 27.79% 1.8 30.14 3.34 28.27 98.94% 61822 DR.TARIQ RASHID 1.25 1.12 9.88% 28.99 -

Das) of Large Cities (Lahore, Faisalabad, Rawalpindi, Gujranwala & Multan) and of Bahawalpur Development Authority (BDA) and Fort Monroe Development Authority (FDMA
URBAN DEVELOPMENT Urban Development Sector covers development projects sponsored by WASAs and Development Authorities (DAs) of large cities (Lahore, Faisalabad, Rawalpindi, Gujranwala & Multan) and of Bahawalpur Development Authority (BDA) and Fort Monroe Development Authority (FDMA). VISION Provision of adequate and efficient urban services in cities, and harnessing their potential for making them engines of economic growth. POLICY Provincial allocation of resources to the WASAs/Development Authorities for the following works/projects keeping in view their population, level of service delivery and keysocio-economic indicators: • Laying of trunk sewers and construction of drains • Construction of new disposal stations • Construction of Sewage Treatment Plants (STP) • Construction of Flyovers, Ring roads, Bypasses and Interchanges OBJECTIVES • Supply of potable drinking water and its efficient use • Provision of effective and efficient sewerage and drainage system • Environment friendly disposal of sewage • Safe and efficient roads infrastructure • Provision of solid waste management services • Strategic planning for growth of cities on scientific lines STRATEGY • A central principle of Urban Strategy is that “density” and “agglomeration” are central to economic development, higher productivity, social equity and human development. To make Punjab competitive for investment and development, cities are going to play a vital role, because they can benefit from a large and skilled labor force, economies of urban scale, economies of agglomeration (i.e. efficiency resulting from clustering of firms in a given 437 industry or related industries), and the resulting demand for goods and services. • Further, rural-urban migration and urbanization can only lead to higher income if manufacturing and services grow fast enough to absorb the supply of labor. -

SHIFA INTERNATIONAL HOSPITALS LIMITED DETAIL of WITHOUT CNIC SHAREHOLDERS S.No
SHIFA INTERNATIONAL HOSPITALS LIMITED DETAIL OF WITHOUT CNIC SHAREHOLDERS S.No. Folio Name Address No. of Shares Net Amount 1 11 DR. SYED JAVED AHMAD 11 STABLE COURT, MUTTON NY-11732, USA. 10,548 17,404 2 31 MRS. M. A. ZAHIR ANSARI 2058 W. FOSTER AVE., CHICAGO, IL-60625, USA. 19,350 31,927 3 32 DR. JAVED I. BANGASH 6 YORKTOWN CT., S. BARRINGTON IL 60010, 150 247 USA. 4 64 DR. MOHAMMAD HAROON BALOCH 4016 EDWARD PRIDE WYND, RALEIGH NC 27612, 20,000 33,000 USA. 5 71 DR. SALMA KAFEEL QURESHI H-10, ST-72, F-8/3, ISLAMABAD. 1,000 1,650 6 72 MR. RASHID AHMAD LODHI 14461 MAISANO DR., STERLING HEIGHTS MI- 20,000 33,000 48312, USA. 7 81 DR. GHULAM ARIF 853 CHERRY LANE, WYCOMBE PA 18980, USA. 60,750 100,237 8 97 DR. ABDUL AZIZ 2540 WASHINGTON STE 601 WAUKEGAN, IL 10,000 16,500 60085, USA. 9 118 MISS ADEALA CHAUDHRY 1/4B, COMMERCIAL AREA, MOHAMMAD ALI 10,000 16,500 SOCIETY, KARACHI. 10 155 MR. MOHAMMED ASIF KHAN SHIFA PHARMACY, 7200 MARKHAM RD # 4, 10,000 16,500 MARKHAM, ONTARIO L3S.3P1 CANADA. 11 182 MR. MOHAMMAD SHABBIR P. O. BOX-10801, JEDDAH-21443, KSA. 10,540 17,391 12 190 MR. SHAKIL BAIG P. O. BOX-10447, ST. THOMAS VI 00801, USA. 136,217 224,758 13 197 MIAN RAFIQ AHMAD 14 SANDAL FOOT DR., POTOMAE, MD-20854, 26,000 42,900 USA. 14 204 MR. MOHAMMED IDRIES TEH: & P.O. KOTLI-SATYIAN, VILLAGE MATEEZ, 400 660 DISTT: RAWALPINDI. -
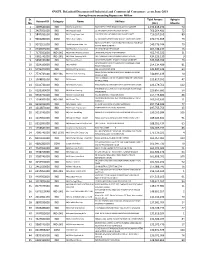
Disconnected Defaulters for Web Site June-19.Xlsx
SNGPL Defaulted Disconnected Industrial and Commercial Consumers as on June-2019 Having Arrears exceeding Rupees one Million Sr. Total Arrears Aging in Account ID Category Name Address No. (Rs.) Months 1 1089641000 IND M/S Tariq Aziz Box FACTORY 510 PECO ROAD KOT LAKHPAT LAHORE 2,193,435,290 74 2 2467051000 IND M/S Yaqoob Steel 32 KM SHEIKHUPURA RD SHEIKHUPURA 766,264,481 72 3 5847051000 IND M/S Flying Paper Inds 103 FAZA ROAD ST JOHN PARK LAHORE CANTT 710,207,165 89 4 9286282000 COM M/S A W S Traders 12 KM SHEIKHUPURA ROAD LIBERTY TOWN SHAHDARA 670,774,859 73 M/S VALUE FUELS C N G FILLING STATION MULTAN ROAD Abdul Qayyum Khan Co 5 9571311000 IND NR PAF BASE MIANWALI 540,776,744 70 6 0390873000 IND M/S Bismillah Corporation I E KOHAT ROAD PESHAWAR 462,748,437 16 7 7173590000 IND-LNG M/S Amtex Private Limited JARANWALA ROAD KHURRIANWALA 452,743,332 21 8 6901741000 IND-LNG M/S Nisar Spinning Mills (PVT) LIMITED 03 KM RAIWIND SUNDER LAHORE 434,226,130 9 9 5450741000 IND M/S Hassan Board INDUSTRIES (PAPER BOARD G T ROAD MURIDKEY 343,595,269 73 WOOD BASED PANELS CHAK NO 67 JB SADHAR JHNAG M/S Makkah 10 3024590000 IND ROAD FAISALABAD 254,134,489 81 11 5995293000 IND M/S Cantt Cng Services MALAKAND ROAD MDN 242,847,124 63 MILLS (POWER GENERATION) 4 KM MANGA RAIWIND M/S Platinum Spinning 12 7720741000 IND-LNG ROAD LAHORE 240,891,433 10 TEXTILE MILLS LTD 3 KM MUDDOKI ROAD NR TOBA ROAD M/S Galaxy 13 1568831000 IND BY PASS JHANG 223,817,551 47 14 6110741000 IND M/S Novelty Fabrics PROCESSING NR CENTURY PAPER JAMBER DISTT KASUR 223,479,762 122 PINISHING