Calcium Antagonists in Migraine Prophylaxis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Phase I Trial of Tamoxifen with Ribociclib (LEE011) in Adult Patients with Advanced ER+ (HER2 Negative) Breast Cancer
The TEEL Study: A Phase I Trial of Tamoxifen with Ribociclib (LEE011) in Adult Patients with Advanced ER+ (HER2 Negative) Breast Cancer NCT02586675 Version 12.0 September 14, 2016 TEEL Protocol- Tamoxifen +Ribociclib Page 1 TITLE PAGE The TEEL Study: A Phase I trial of Tamoxifen with Ribociclib (LEE011) in adult patients with advanced ER+ (HER2 negative) breast cancer. Protocol: MCC 18332 Chesapeake IRB Pro00015228 Principal Investigator: Co-Investigators: Statistician: Experimental Therapeutics Program H. Lee Moffitt Cancer Center 12902 Magnolia Drive Tampa, FL 33612 & Comprehensive Breast Program Moffitt McKinley Outpatient Center 10920 N. McKinley Dr. Tampa, FL 33612 Study Site Contact: Protocol Version 12 Date: September 14, 2016 TEEL Protocol- Tamoxifen +Ribociclib Page 2 TITLE PAGE .............................................................................................................................................. 1 SYNOPSIS ................................................................................................................................................... 5 Patient Population ................................................................................................................................. 5 Type of Study ......................................................................................................................................... 5 Prior Therapy......................................................................................................................................... 5 -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

Dorset Medicines Advisory Group
DORSET CARDIOLOGY WORKING GROUP GUIDELINE FOR CALCIUM CHANNEL BLOCKERS IN HYPERTENSION SUMMARY The pan-Dorset cardiology working group continues to recommend the use of amlodipine (a third generation dihydropyridine calcium-channel blocker) as first choice calcium channel blocker on the pan-Dorset formulary for hypertension. Lercanidipine is second choice, lacidipine third choice and felodipine is fourth choice. This is due to preferable side effect profiles in terms of ankle oedema and relative costs of the preparations. Note: where angina is the primary indication or is a co-morbidity prescribers must check against the specific product characteristics (SPC) for an individual drug to confirm this is a licensed indication. N.B. Lacidipine and lercandipine are only licensed for use in hypertension. Chapter 02.06.02 CCBs section of the Formulary has undergone an evidence-based review. A comprehensive literature search was carried out on NHS Evidence, Medline, EMBASE, Cochrane Database, and UK Duets. This was for recent reviews or meta-analyses on calcium channel blockers from 2009 onwards (comparative efficacy and side effects) and randomised controlled trials (RCTs). REVIEW BACKGROUND Very little good quality evidence exists. No reviews, meta-analyses or RCTs were found covering all calcium channel blockers currently on the formulary. Another limitation was difficulty obtaining full text original papers for some of the references therefore having to use those from more obscure journals instead. Some discrepancies exist between classification of generations of dihydropyridine CCBs, depending upon the year of publication of the reference/authors’ interpretation. Dihydropyridine (DHP) CCBs tend to be more potent vasodilators than non-dihydropyridine (non-DHP) CCBs (diltiazem, verapamil), but the latter have greater inotropic effects. -

Current and Prospective Pharmacological Targets in Relation to Antimigraine Action
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Erasmus University Digital Repository Naunyn-Schmiedeberg’s Arch Pharmacol (2008) 378:371–394 DOI 10.1007/s00210-008-0322-7 REVIEW Current and prospective pharmacological targets in relation to antimigraine action Suneet Mehrotra & Saurabh Gupta & Kayi Y. Chan & Carlos M. Villalón & David Centurión & Pramod R. Saxena & Antoinette MaassenVanDenBrink Received: 8 January 2008 /Accepted: 6 June 2008 /Published online: 15 July 2008 # The Author(s) 2008 Abstract Migraine is a recurrent incapacitating neuro- (CGRP1 and CGRP2), adenosine (A1,A2,andA3), glutamate vascular disorder characterized by unilateral and throbbing (NMDA, AMPA, kainate, and metabotropic), dopamine, headaches associated with photophobia, phonophobia, endothelin, and female hormone (estrogen and progesterone) nausea, and vomiting. Current specific drugs used in the receptors. In addition, we have considered some other acute treatment of migraine interact with vascular receptors, targets, including gamma-aminobutyric acid, angiotensin, a fact that has raised concerns about their cardiovascular bradykinin, histamine, and ionotropic receptors, in relation to safety. In the past, α-adrenoceptor agonists (ergotamine, antimigraine therapy. Finally, the cardiovascular safety of dihydroergotamine, isometheptene) were used. The last two current and prospective antimigraine therapies is touched decades have witnessed the advent of 5-HT1B/1D receptor upon. agonists (sumatriptan and second-generation triptans), which have a well-established efficacy in the acute Keywords 5-HT. Antimigraine drugs . CGRP. treatment of migraine. Moreover, current prophylactic Noradrenaline . Migraine . Receptors treatments of migraine include 5-HT2 receptor antagonists, Ca2+ channel blockers, and β-adrenoceptor antagonists. Despite the progress in migraine research and in view of its Introduction complex etiology, this disease still remains underdiagnosed, and available therapies are underused. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Preventive Report Appendix
Title Authors Published Journal Volume Issue Pages DOI Final Status Exclusion Reason Nasal sumatriptan is effective in treatment of migraine attacks in children: A Ahonen K.; Hamalainen ML.; Rantala H.; 2004 Neurology 62 6 883-7 10.1212/01.wnl.0000115105.05966.a7 Deemed irrelevant in initial screening Seasonal variation in migraine. Alstadhaug KB.; Salvesen R.; Bekkelund SI. Cephalalgia : an 2005 international journal 25 10 811-6 10.1111/j.1468-2982.2005.01018.x Deemed irrelevant in initial screening Flunarizine, a calcium channel blocker: a new prophylactic drug in migraine. Amery WK. 1983 Headache 23 2 70-4 10.1111/j.1526-4610.1983.hed2302070 Deemed irrelevant in initial screening Monoamine oxidase inhibitors in the control of migraine. Anthony M.; Lance JW. Proceedings of the 1970 Australian 7 45-7 Deemed irrelevant in initial screening Prostaglandins and prostaglandin receptor antagonism in migraine. Antonova M. 2013 Danish medical 60 5 B4635 Deemed irrelevant in initial screening Divalproex extended-release in adolescent migraine prophylaxis: results of a Apostol G.; Cady RK.; Laforet GA.; Robieson randomized, double-blind, placebo-controlled study. WZ.; Olson E.; Abi-Saab WM.; Saltarelli M. 2008 Headache 48 7 1012-25 10.1111/j.1526-4610.2008.01081.x Deemed irrelevant in initial screening Divalproex sodium extended-release for the prophylaxis of migraine headache in Apostol G.; Lewis DW.; Laforet GA.; adolescents: results of a stand-alone, long-term open-label safety study. Robieson WZ.; Fugate JM.; Abi-Saab WM.; 2009 Headache 49 1 45-53 10.1111/j.1526-4610.2008.01279.x Deemed irrelevant in initial screening Safety and tolerability of divalproex sodium extended-release in the prophylaxis of Apostol G.; Pakalnis A.; Laforet GA.; migraine headaches: results of an open-label extension trial in adolescents. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Suspension D'amm Des Médicaments Par Voie Orale Contenant
INFORMATION TRANSMISE SOUS L’AUTORITE DE L’ANSM Lettre aux professionnels de Santé Septembre 2013 Communication aux professionnels de santé concernant les restrictions d’indications des médicaments par voie orale contenant : dihydroergotamine, dihydroergocristine, dihydroergocryptine-caféine, nicergoline A destination des : neurologues, ophtalmologistes, cardiologues, chirurgiens vasculaires, phlébologues, angéiologues, gériatres, médecins généralistes, pharmaciens (ville + hôpital) , CRPV Madame, Monsieur, Cher Confrère, En accord avec l’Agence Nationale de Sécurité du Médicament et des produits de santé (ANSM) et l’Agence européenne du médicament (EMA), nous vous informons que les médicaments contenant de la dihydroergotamine, de la dihydroergocristine, de la dihydroergocryptine-caféine ou de la nicergoline ne devront plus être utilisés dans les indications suivantes : Dihydroergotamine (SEGLOR, TAMIK, IKARAN, DIHYDROERGOTAMINE AMDIPHARM): • Traitement de fond de la migraine. • Traitement de l’hypotension orthostatique. • Amélioration des symptômes en rapport avec l'insuffisance veinolymphatique (jambes lourdes, douleurs, impatience du primo-decubitus). Dihydroergocristine (ISKEDYL): • Traitement à visée symptomatique du déficit pathologique cognitif et neurosensoriel chronique du sujet âgé (à l’exclusion de la maladie d’Alzheimer et des autres démences). • Traitement d’appoint des baisses d’acuité visuelle et troubles présumés du champ visuel d’origine vasculaire. • Rétinopathies aigües d’origine vasculaire Dihydroergocryptine-caféine (VASOBRAL) : • Traitement d'appoint à visée symptomatique du déficit pathologique cognitif et neurosensoriel chronique du sujet âgé (à l'exclusion de la maladie d'Alzheimer et des autres démences). • Traitement d'appoint du syndrome de Raynaud. Nicergoline (SERMION, NICERGOLINE BIOGARAN, NICERGOLINE EG, NICERGOLINE MYLAN, NICERGOLINE TEVA): • Traitement d'appoint à visée symptomatique du déficit pathologique cognitif et neurosensoriel chronique du sujet âgé (à l'exclusion de la maladie d'Alzheimer et des autres démences). -
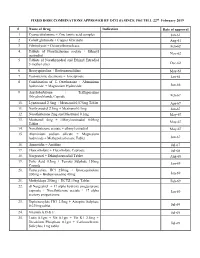
FIXED DOSE COMBINATIONS APPROVED by DCG (I) SINCE 1961 TILL 22Nd February 2019
FIXED DOSE COMBINATIONS APPROVED BY DCG (I) SINCE 1961 TILL 22nd February 2019 # Name of Drug Indication Date of approval 1. Cyanocobalamine + Zinc tannic acid complex Jan-61 2. Cobalt glutamate + Copper Glycinate Aug-61 3. Fibrinolysin + Desoxyribonuclease Feb-62 4. Tablets of Norethisterone acetate + Ethinyl Nov-62 oestradiol 5. Tablets of Norethynodrel and Ethinyl Estradiol 3-methyl ether Dec-62 6. Broxyquinoline + Brobenzoxalidine May-63 7. Testosterone decanoate + Isocaproate Jan-64 8. Combination of L Oxethazaine + Aluminium hydroxide + Magnesium Hydroxide Jun-66 9. Amylobarbitone + Trifluperazine Dihydrochloride Capsule Feb-67 10. Lynestronol 2.5mg + Mestranol 0.075mg Tablet Apr-67 11. Northynodrel 2.5mg + Mestranol 0.1mg Jun-67 12. Norethisterone 2mg and Mestranol 0.1mg May-67 13. Mestranol 4mg + Ethinyloestradial 0.05mg May-67 Tablet 14. Norethisterane acetate + ethinyl estradiol May-67 15. Aluminium sodium silicate + Magnesium hydroxide + Methypolysiloxane Tablet Jun-67 16. Ammoidin + Amidine Jul-67 17. Fluocortolene + Flucortolene Caproate Jul-68 18. Norgestrel + Ethinyloestradiol Tablet Aug-68 19. Folic Acid 0.5mg + Ferrous Sulphate 150mg Jan-69 Capsule 20. Tetracycline HCl 250mg + Broxyquinoline 200mg + Brobenzoxadine 40mg Jan-69 21. Methyldopa 250mg + HCTZ 15mg Tablet Feb-69 22. dl Norgestrel + 17 alpha hydroxy progesterone caproate + Norethisterone acetate + 17 alpha Jan-69 acetoxy progesterone 23. Diphenoxylate HCl 2.5mg + Atropine Sulphate 0.025mg tablet Jul-69 24. Vitamin A,D & E Jul-69 25. Lutin 0.1gm + Vit 0.1gm + Vit K1 2.5mg + Dicalcium Phosphate 0.1gm + Carlozochrome Jul-69 Salicylate 1mg tablet 26. Vit K 1 5mg + Calcium Lactolionate 100 m g+ Carlozocrome Salicylate 2.5mg + Phenol 0.5% Jul-69 + Lignocaine Hcl 1% injection 27. -

Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Cr
Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Crizotinib (PF-02341066) 1 4 55 Docetaxel 1 5 98 Anastrozole 1 6 25 Cladribine 1 7 23 Methotrexate 1 8 -187 Letrozole 1 9 65 Entecavir Hydrate 1 10 48 Roxadustat (FG-4592) 1 11 19 Imatinib Mesylate (STI571) 1 12 0 Sunitinib Malate 1 13 34 Vismodegib (GDC-0449) 1 14 64 Paclitaxel 1 15 89 Aprepitant 1 16 94 Decitabine 1 17 -79 Bendamustine HCl 1 18 19 Temozolomide 1 19 -111 Nepafenac 1 20 24 Nintedanib (BIBF 1120) 1 21 -43 Lapatinib (GW-572016) Ditosylate 1 22 88 Temsirolimus (CCI-779, NSC 683864) 1 23 96 Belinostat (PXD101) 1 24 46 Capecitabine 1 25 19 Bicalutamide 1 26 83 Dutasteride 1 27 68 Epirubicin HCl 1 28 -59 Tamoxifen 1 29 30 Rufinamide 1 30 96 Afatinib (BIBW2992) 1 31 -54 Lenalidomide (CC-5013) 1 32 19 Vorinostat (SAHA, MK0683) 1 33 38 Rucaparib (AG-014699,PF-01367338) phosphate1 34 14 Lenvatinib (E7080) 1 35 80 Fulvestrant 1 36 76 Melatonin 1 37 15 Etoposide 1 38 -69 Vincristine sulfate 1 39 61 Posaconazole 1 40 97 Bortezomib (PS-341) 1 41 71 Panobinostat (LBH589) 1 42 41 Entinostat (MS-275) 1 43 26 Cabozantinib (XL184, BMS-907351) 1 44 79 Valproic acid sodium salt (Sodium valproate) 1 45 7 Raltitrexed 1 46 39 Bisoprolol fumarate 1 47 -23 Raloxifene HCl 1 48 97 Agomelatine 1 49 35 Prasugrel 1 50 -24 Bosutinib (SKI-606) 1 51 85 Nilotinib (AMN-107) 1 52 99 Enzastaurin (LY317615) 1 53 -12 Everolimus (RAD001) 1 54 94 Regorafenib (BAY 73-4506) 1 55 24 Thalidomide 1 56 40 Tivozanib (AV-951) 1 57 86 Fludarabine