Hunting with Catapults: the Predatory Strike of the Dragonfly Larva
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adventuring with Books: a Booklist for Pre-K-Grade 6. the NCTE Booklist
DOCUMENT RESUME ED 311 453 CS 212 097 AUTHOR Jett-Simpson, Mary, Ed. TITLE Adventuring with Books: A Booklist for Pre-K-Grade 6. Ninth Edition. The NCTE Booklist Series. INSTITUTION National Council of Teachers of English, Urbana, Ill. REPORT NO ISBN-0-8141-0078-3 PUB DATE 89 NOTE 570p.; Prepared by the Committee on the Elementary School Booklist of the National Council of Teachers of English. For earlier edition, see ED 264 588. AVAILABLE FROMNational Council of Teachers of English, 1111 Kenyon Rd., Urbana, IL 61801 (Stock No. 00783-3020; $12.95 member, $16.50 nonmember). PUB TYPE Books (010) -- Reference Materials - Bibliographies (131) EDRS PRICE MF02/PC23 Plus Postage. DESCRIPTORS Annotated Bibliographies; Art; Athletics; Biographies; *Books; *Childress Literature; Elementary Education; Fantasy; Fiction; Nonfiction; Poetry; Preschool Education; *Reading Materials; Recreational Reading; Sciences; Social Studies IDENTIFIERS Historical Fiction; *Trade Books ABSTRACT Intended to provide teachers with a list of recently published books recommended for children, this annotated booklist cites titles of children's trade books selected for their literary and artistic quality. The annotations in the booklist include a critical statement about each book as well as a brief description of the content, and--where appropriate--information about quality and composition of illustrations. Some 1,800 titles are included in this publication; they were selected from approximately 8,000 children's books published in the United States between 1985 and 1989 and are divided into the following categories: (1) books for babies and toddlers, (2) basic concept books, (3) wordless picture books, (4) language and reading, (5) poetry. (6) classics, (7) traditional literature, (8) fantasy,(9) science fiction, (10) contemporary realistic fiction, (11) historical fiction, (12) biography, (13) social studies, (14) science and mathematics, (15) fine arts, (16) crafts and hobbies, (17) sports and games, and (18) holidays. -

KOONTZ, Dean R(Ay)
KOONTZ, Dean R (ay) Geboren: Everett, Pennsylvania, USA, 9 juli 1945 Pseudoniemen: David Axton; Brian Coffey, Deanna Dwyer; K.R. Dwyer; John Hill; Leigh Nichols; Anthony North; Richard Paige; Owen West Opleiding: Shippensburg State College, Pennsylvania, B.A. engels, 1966 Carrière: werkte bij een federaal armoede-bestrijdings programma, 1966-1967; leraar engels op een high school, 1967-1969; full-time schrijver sedert 1969. Familie: getrouwd met Gerda Ann Cerra, 1966 Woont in Orange, Californië. (foto: Fantastic Fiction) Detective: Michael Tucker (o.ps. Brian Coffey) detective: Odd Thomas kan de doden zien en ziet in zijn dromen wat mensen te wachten staat. Odd Thomas: 1. Odd Thomas 2003 De gave 2004 Luitingh ook verschenen als filmeditie odt: Odd Tomas: De gave 2015 Luitingh 2. Forever Odd 2005 De vriendschap 2006 Luitingh 3. Brother Odd 2006 De broeder 2007 Luitingh 4. Odd Hours 2008 De ziener 2008 Luitingh 5. Odd Apocalypse 2012 De miljardair 2012 Luitingh 6. Deeply Odd 2013 De lifter 2013 Luitingh~Sijthoff 7. Saint Odd 2015 graphic novels: In Odd We Trust #12 2008 Odd Is on Our Side #13 2010 House of Odd #14 2012 novellas: 1. Odd Interlude Part One 2012 2. Odd Interlude Part Two 2012 3. Odd Interlude Part Three 2012 4. Odd Interlude 2012 Het motel 2013 Luitingh~Sijthoff omslag ondertitel: A Special Odd Thomas Adventure 5. You Are Destined to Be Together Forever 2014 omnibus: The Complete Thomas Odd Series 2016 bevat: Odd Thomas Forever Odd Brother Odd Odd Hours Odd Apocalypse Odd Interlude Deeply Odd Saint Odd andere crimetitels: -

The Crimson Witch
The Crimson Witch by Dean Koontz THE MANBAT CHITTERED LOUDLY IN A SAVAGE WAR HOOPlifted itself with beating wings, raked its claws down Jake's cheeks. The second manbat swept in and was upon him. He swiped feebly with his knife, but all his strength had left him. He could barely swallow the blood as fast as it poured into his mouth. The manbats screamed wildly with knowledge of their success, then headed for his eyes THE CRIMSON WITCH BY DEAN R. KOONTZ MODERN LITERARY EDITIONS PUBLISHING COMPANY NEW YORK, N.Y. Copyright Š 1971 by Dean R. Koontz PRINTED IN THE UNITED STATES OF AMERICA All Rights Reserved Prologue: THE CRIMSON WITCH She came spinning out of the thunderstorm, mad as all hell. Lightning flashed above her, rippled across the horizon like a great, semitransparent jellyfish, sinking liquidly into the horizon. The sky was a uniform gun-metal gray as if the clouds had been hammered into sheets and welded together from horizon to horizon by some industrious God of Melancholy. Thunder boom-aboomed like mountainous waves crashing against weathered rocks, each clap trailing off into the whisper of seafoam. Boom! Ssshusscrack! Her anger boiled as fiercely as the elements, lanced through her mind in awesome, painful flashes. Her red robes fluttered behind her as she drifted through the night, swept in a halo like satin wings, filtered the lightning into the color of freshly spilled blood. She plunged into the dank, heavy clouds and came out in the spaces between, unruffled. Following the pulsations of the mammoth storm, she moved downward toward the small and fearful earth. -

Learning from Science Fiction
HARD READING Liverpool Science Fiction Texts and Studies, 53 Liverpool Science Fiction Texts and Studies Editor David Seed, University of Liverpool Editorial Board Mark Bould, University of the West of England Veronica Hollinger, Trent University Rob Latham, University of California Roger Luckhurst, Birkbeck College, University of London Patrick Parrinder, University of Reading Andy Sawyer, University of Liverpool Recent titles in the series 30. Mike Ashley Transformations: The Story of the Science-Fiction Magazine from 1950–1970 31. Joanna Russ The Country You Have Never Seen: Essays and Reviews 32. Robert Philmus Visions and Revisions: (Re)constructing Science Fiction 33. Gene Wolfe (edited and introduced by Peter Wright) Shadows of the New Sun: Wolfe on Writing/Writers on Wolfe 34. Mike Ashley Gateways to Forever: The Story of the Science-Fiction Magazine from 1970–1980 35. Patricia Kerslake Science Fiction and Empire 36. Keith Williams H. G. Wells, Modernity and the Movies 37. Wendy Gay Pearson, Veronica Hollinger and Joan Gordon (eds.) Queer Universes: Sexualities and Science Fiction 38. John Wyndham (eds. David Ketterer and Andy Sawyer) Plan for Chaos 39. Sherryl Vint Animal Alterity: Science Fiction and the Question of the Animal 40. Paul Williams Race, Ethnicity and Nuclear War: Representations of Nuclear Weapons and Post-Apocalyptic Worlds 41. Sara Wasson and Emily Alder, Gothic Science Fiction 1980–2010 42. David Seed (ed.), Future Wars: The Anticipations and the Fears 43. Andrew M. Butler, Solar Flares: Science Fiction in the 1970s 44. Andrew Milner, Locating Science Fiction 45. Joshua Raulerson, Singularities 46. Stanislaw Lem: Selected Letters to Michael Kandel (edited, translated and with an introduction by Peter Swirski) 47. -
Courage & Determination
116 East Woodin Ave. PO Box 686 Chelan WA 98816 “Lake Chelan Valley’s Community Bookstore since 1994” Summer 2010 Phone: 509.682.8901 Courage & Determination Island Beneath the Sea Girl in Translation Born a slave on the island When Kimberly Chang and of Saint-Domingue, T’t’ her mother emigrate from is the daughter of an Hong Kong to Brooklyn African mother she never squalor, she quickly begins a Visit us at the foot of Lake knew and one of the white secret double life: exceptional Chelan, a 55 mile long sailors who brought her schoolgirl during the day, glacier-fed lake, surrounded into bondage. When twenty- Chinatown sweatshop worker by the Cascade Mountains year-old Toulouse Valmorain in the evenings. Disguising the and the Columbia River. arrives on the island in 1770 more diffi cult truths of her life – Two hour parking is to run his father’s plantation, like the staggering degree of her available on city streets or, park in one of the three he fi nds it neither glamorous poverty, the weight of her family’s parking lots in town. nor easy. It will be eight years future resting on her shoulders, before he brings home a bride – but marriage, too, proves or her secret love for a factory boy who shares none of her Store Hours more diffi cult than he imagined. So Valmorain remains talent or ambition – Kimberly learns to constantly translate MONDAY -SATURDAY 9 TO 6 dependent on the services of T’t’, his teenaged slave, not just her language but herself back and forth between SUNDAY 10 TO 5 for everything from coerced sex to caring for his wife the worlds she straddles. -

Silent Corner Discussion Questions by Dean Koontz
Silent Corner Discussion Questions by Dean Koontz Author Bio: (from Fantastic Fiction & Dean Koontz website) Dean Koontz is acknowledged as one of today's most celebrated and successful writers. His books are published in 38 languages and he has sold over 500 million copies to date. Dean Koontz lives in Southern California with his wife, Gerda, their golden retriever, Elsa, and the enduring spirit of their goldens, Trixie and Anna. Characters: Jane Hawk – Former FBI agent. Used to provide investigative support for the FBI Behavioral Science Unit. Widowed four months ago. Her husband, Nick, was a Marine. They have a son, Travis (5). Her mother is deceased. Her father is a famous musician. Mr. Drood – The alias of the man/group threatening Jane and Travis. Name taken from “A Clockwork Orange.” Kipp Garner – Radburn’s partner/henchman who enjoys violence. John Harrow – Special Agent in Charge of the LA FBI office. Nick Hawk – Former Marine. Unexpectedly and uncharacteristically kills himself with a knife. Married to Jane Hawk. Booth Hendrickson – Works in the Department of Justice. Becomes Silverman’s handler after his infection. Randolph Kohl – Director of Homeland Security. Gwyneth Lambert – Widow. Her husband, Gordon, was a former marine Lieutenant General who committed suicide. David James Michael – On the board of the Gernsback institute (What if Conference). Inherited a fortune. Venture capitalist who funds high tech startups – Shenneck’s technology and Far Horizons. William Sterling Overton – Lawyer. Friend/works for Senneck. Member of Aspasia. Jimmy Radburn – Hacker/cracker extraordinaire. Thinks of himself as a cyberspace pirate. His real name is Robert Branwick. -

Bibliography of Reviews of the Works for Dean Koontz As Removed from the Manuscript of the Collectors Guide to Dean Koontz by Michael P
A (very incomplete) Bibliography of reviews of the works for Dean Koontz as removed from the manuscript of The Collectors Guide to Dean Koontz by Michael P. Sauers to be published by Cemetery Dance Publications in 2011 The story behind this document can be found @ http://www.travelinlibrarian.info/CGTDK/2010/08/12/review-removal/ After the Last Race by Dean R. Koontz Reviews Kirkus Reviews v42 Sept. 1, 1974 p961 Publisher’s Weekly v206 Sept. 23, 1974 p148 Library Journal v99 Oct. 1, 1974 p2505 NEW YORK TIME BOOK REVIEW Jan. 12,1975 p18 Best Sellers v34 Feb. 1, 1975 p498 Publisher’s Weekly v208 Nov. 17, 1975 p99 Anti-Man by Dean R. Koontz Reviews Luna Monthly 26/27:33 Jl/Aug 1971 -Evers, J. The Bad Place by Dean R. Koontz Reviews New York Times Feb. 08,1989 pC26 -McDowell, E. Kirkus Reviews v57 Nov. 1, 1989 p1551 Booklist v86 Nov. 15, 1989 p618 Publisher’s Weekly Nov. 24,1989 p59 -Steinberg, S. Library Journal v114 Dec 1989 p170 (2) -Annichiarico, Mark Connoisseur v219 Dec 1989 p40 -Sawhill, Ray Rave Reviews #22, Dec/Jan (1989/90) p71 -Frank A. LaPorto The Blood Review v1 #2, Jan 1990 p51 -Richard Wellgosh The Blood Review v1 #2, Jan 1990 p51 -Mark Grahm West Coast Review of Books v15 #3 1990 p29 Atlanta Journal-Constitution Jan 21 1990 sec N p8 -Janet Ward LA Times Book Review Jan. 21, 1990 p12 Boston Globe Jan 25 1990 p75 -Bob MacDonald San Francisco Chronicle Feb 4 1990 sec REV p6 -Leila Raim New York Times Book Review Feb. -

Dragonfly Monsoon’ and Imagined Oceans: in Search of Poem-Maps of the Swahili Seas
‘Dragonfly Monsoon’ and Imagined Oceans: In Search of Poem-Maps of the Swahili Seas Yvonne Adhiambo Owuor M.A. University of Reading, UK B.A. Kenyatta University, Kenya A thesis submitted for the degree of Master of Philosophy at The University of Queensland in 2014 The School of Communications and Arts Abstract To what extent can a ‘text’ borne in memory give texture, perspectives, meaning, dimensionality and sense to a place? Where is the ‘locus of meaning’ in such a case? Can this locus be traced (mapped, narrated, found)? These questions about Swahili navigational poetry (poem-maps) specific to the Western Indian Ocean first motivated this study. Can such repositories of sea experiences be read for biographical threads of the water body to which they refer? As the search for answers to these questions progressed in the course of the study, it emerged that studies of the Western Indian Ocean’s intimate and imagined geographies through the lives and memories – individual and collective, private and institutional - of those who have had the closest and most extensive relationship with it were not, at present, easily available. The quest turned to narratives in and of the interstices, the ‘small stories’ (micro-narratives), those seemingly ordinary tales of encounter and experience that get obliterated in the retelling of overarching historical (‘China in Africa’) and geographical (‘The Indian Ocean’) phenomena. This thesis, Dragonfly Monsoon (novel excerpt) and Imagined Oceans: in search of poem-maps of the Swahili Seas, becomes an effort to story the ocean. The project does this by drawing from the memories and selected sea poetry of Zanzibar-based Haji Gora Haji, a living mariner, and the maritime lives of fictional mariners who, with Haji Gora Haji, inhabit these shores and waters. -
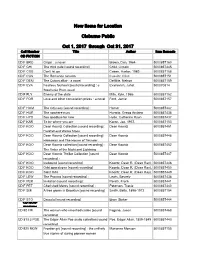
New Items for Location Cleburne Public Oct 1, 2017 Through Oct 31
New Items for Location Cleburne Public Oct 1, 2017 through Oct 31, 2017 Call Number Title Author Item Barcode CD FICTION CD F BRO Origin : a novel Brown, Dan, 1964- 5000857160 CD F CHI The third gate [sound recording] Child, Lincoln 5000857445 CD F COB Don't let go Coben, Harlan, 1962- 5000857158 CD F CUS The Romanov ransom Cussler, Clive 5000857151 CD F DEM The Cuban affair : a novel DeMille, Nelson 5000857159 CD F EVA Fearless fourteen [sound recording] : a Evanovich, Janet 500070814 Stephanie Plum novel CD F FLY Enemy of the state Mills, Kyle, 1966- 5000857152 CD F FOR Love and other consolation prizes : a novel Ford, Jamie 5000857157 CD F HOM The Odyssey [sound recording] : Homer 5000857442 CD F HUR The nowhere man Hurwitz, Gregg Andrew 5000857436 CD F HYD Say goodbye for now Hyde, Catherine Ryan 5000857437 CD F KAR To be where you are Karon, Jan, 1937- 5000857153 CD F KOO Dean Koontz Collection [sound recording] : Dean Koontz 5000857451 Darkfall and Winter Moon CD F KOO Dean Koontz Collection [sound recording] : Dean Koontz 5000857448 Hideaway and The House of Thunder CD F KOO Dean Koontz collection [sound recording] : Dean Koontz 5000857452 The Voice of the Night and Lightning CD F KOO Dean Koontz Thriller Collection [sound Dean Koontz 5000857447 recording] : CD F KOO Icebound [sound recording] Koontz, Dean R. (Dean Ray), 5000857446 CD F KOO Odd apocalypse [sound recording] Koontz, Dean R. (Dean Ray), 5000857450 CD F KOO Saint Odd Koontz, Dean R. (Dean Ray), 5000857449 CD F LEW The Proving [sound recording] : Lewis, Beverly 5000857438 CD F PER Invitation [sound recording] : Peretti, Frank 5000857441 CD F PET Cherished Mercy [sound recording] : Peterson, Tracie 5000857440 CD F SMI A tree grows in Brooklyn [sound recording] Smith, Betty, 1896-1972 5000857154 CD F STO Dracula [sound recording] Bram Stoker 5000857444 CD NON- FICTION CD 92 FRI The woman who smashed codes [sound Fagone, Jason 5000857468 recording] CD 813.3 POE The Edgar Allan Poe audio collection [sound Poe, Edgar Allan, 1809-1849 5000857443 recording].