Ulcerative Colitis Treatment Options Brochure | ENTYVIO (Vedolizumab)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Positioning Biologics in the Management of Moderate to Severe Crohn's Disease
REVIEW CURRENT OPINION Positioning biologics in the management of moderate to severe Crohn’s disease Jana G. Hashash and Fadi H. Mourad Purpose of review Since there is a lack of head-to-head randomized controlled trials, little direction is provided from guidelines on the positioning of biologics for the treatment of Crohn’s disease (CD). This review utilizes comparative effectiveness and safety results from real-world data and network meta-analyses to inform clinical practice for positioning of biological therapies in the treatment of moderate-to-severe CD. Recent findings We summarize the results of studies pertaining to the identification of predictors for response to biologics in CD. Recently published studies about the management of moderate-to-severe CD are discussed and a positioning algorithm is proposed for the therapeutic approach of these patients. Summary Different classes of biologics are comparable with regards to safety and almost similar in effectiveness in the management of CD. There are certain clinical scenarios in which one biologic is more effective than another. For instance, patients with a more aggressive disease phenotype such as fistulizing disease would benefit from infliximab over other biologics, whereas in older patients at a higher risk for infectious complications, it may be more appropriate to use ustekinumab or vedolizumab over the anti-tumor necrosis factor (TNF) agents. More data pertaining to identifying predictors of response to the different available therapies and head-to-head comparison trials are needed to personalize our therapeutic approach of CD patients. Keywords biologics, Crohn’s disease, moderate-to-severe, positioning INTRODUCTION The medical management of patients with CD To guide the choice of treatment in patients with has changed over recent years. -

B.C. Pharmacare Drug Information Sheet for Vedolizumab (Entyvio)
The drug below is being considered for possible coverage under the B.C. PharmaCare program. PharmaCare is a government-funded drug plan that helps British Columbians with the cost of eligible prescription drugs and specific medical supplies. For more information on PharmaCare, visit Ministry of Health - PharmaCare. PharmaCare reviews each drug for treating a specific illness or medical condition (known as an “indication”). If a decision is made to cover the drug, it will be only for that illness or condition. In some cases, PharmaCare may cover a drug only for people who have the illness or condition and have not responded to other drugs used to treat that illness or condition. For more information on PharmaCare’s drug coverage review process, see the last page of this information sheet. Information about the drug Generic name (scientific name) vedolizumab Brand name Entyvio® Manufacturer Takeda Canada Inc. Indication Entyvio is indicated for the treatment of moderately to severely active Crohn’s disease in adult patients. Has the drug been reviewed by Yes the Common Drug For more information about the CDR’s review of vedolizumab (Entyvio®), you can Review (CDR)? Search the CDR Reports. (see the note below this table.) Public input start date Friday, November 13, 2020 Public input closing date Friday, December 11, 2020 AT MIDNIGHT How is the drug taken? Entyvio is administered by subcutaneous injection (into the skin). How often is the drug taken? Entyvio is administered every two weeks, following at least two intravenous infusions (into the vein). Ministry of Health Pharmaceutical Services Division Page 1 of 4 BC PharmaCare Drug Information — vedolizumab (Entyvio®) continued… Information about the drug General drug and/or drug Crohn’s disease is a chronic inflammatory disease of the gastrointestinal tract, which study information most commonly affects the small intestine, beginning of large intestine, and rectum. -

Risk Assessment and Risk Mitigation Review(S)
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125476Orig1s000 RISK ASSESSMENT and RISK MITIGATION REVIEW(S) Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Office of Surveillance and Epidemiology Office of Medication Error Prevention and Risk Management Final Risk Evaluation and Mitigation Strategy (REMS) Review Date: May 7, 2014 Reviewer(s): George Neyarapally, Pharm.D., M.P.H. Division of Risk Management (DRISK) Team Leader: Reema Mehta, Pharm.D., M.P.H. DRISK Division Director: Claudia Manzo, Pharm.D. DRISK Drug Name(s): Entyvio (vedolizumab) Therapeutic Class: Lymphocyte α4β7 integrin receptor antagonist Dosage and Route: Solution for infusion Application Type/Number: BLA 125476, BLA 125507 Applicant/Sponsor: Takeda Pharmaceuticals OSE RCM #: 2013-1641 *** This document contains proprietary and confidential information that should not be released to the public. *** 1 Reference ID: 3502774 CONTENTS 1 INTRODUCTION ....................................................................................................... 1 1.1 Product Background............................................................................................ 1 1.2 Disease Background............................................................................................ 2 1.3 Regulatory History .............................................................................................. 2 2 MATERIALS REVIEWED ....................................................................................... -

Datasheet: HCA295 Product Details
Datasheet: HCA295 Description: HUMAN ANTI VEDOLIZUMAB Specificity: VEDOLIZUMAB Other names: Entyvio Format: Purified Product Type: Monoclonal Antibody Clone: AbD30139_hIgG1 Isotype: IgG1 Quantity: 0.1 mg Product Details Applications This product has been reported to work in the following applications. This information is derived from testing within our laboratories, peer-reviewed publications or personal communications from the originators. Please refer to references indicated for further information. For general protocol recommendations, please visit www.bio-rad-antibodies.com/protocols. Yes No Not Determined Suggested Dilution ELISA Where this product has not been tested for use in a particular technique this does not necessarily exclude its use in such procedures. Suggested working dilutions are given as a guide only. It is recommended that the user titrates the product for use in their own system using appropriate negative/positive controls. Product Form Human IgG1 antibody (lambda light chain) selected from the HuCAL® phage display library and expressed in a human cell line. This antibody is supplied as a liquid. Preparation Purified IgG prepared by affinity chromatography on Protein A Buffer Solution Phosphate buffered saline Preservative 0.01% Thiomersal Stabilisers Approx. Protein Antibody concentration 0.5 mg/ml Concentrations Immunogen Vedolizumab Specificity Human Anti-Vedolizumab Antibody, AbD30139_hIgG1 is a paratope specific, inhibitory anti-idiotypic antibody that specifically recognizes the monoclonal antibody drug vedolizumab. The antibody does not recognize free recombinant human α4β7 integrin or vedolizumab in complex with α4β7 integrin and can be used to measure free vedolizumab levels in serum from patients. Clone AbD30139_hIgG1 is a fully human recombinant monoclonal antibody with IgG1 isotype and is suitable as a reference standard in an anti-drug antibody (ADA) assay. -

Entyvio (Vedolizumab) C6773-A R
Prior Authorization Criteria Entyvio (vedolizumab) Policy Number: C6773-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DATE 09/2014 03/28/2019 03/2/2020 J CODE TYPE OF CRITERIA LAST P&T APPROVAL J3380 Inj, vedolizumab, 1 mg RxPA Q2 2019 PRODUCTS AFFECTED: Entyvio (vedolizumab) DRUG CLASS: Integrin Receptor Antagonists ROUTE OF ADMINISTRATION: Intravenous PLACE OF SERVICE: Specialty Pharmacy or Buy and Bill AVAILABLE DOSAGE FORMS: Entyvio SOLR 300MG (300MG Intravenous Solution) FDA-APPROVED USES: Crohn Disease: Treatment of moderately to severely active Crohn disease in patients who have had an inadequate response with, lost response to, or were intolerant to a tumor necrosis factor-alpha (TNF-alpha) blocker or immunomodulator; or had an inadequate response with, were intolerant to, or demonstrated dependence on corticosteroids. Ulcerative Colitis: Treatment of moderately to severely active ulcerative colitis in patients who have had an inadequate response with, lost response to, were intolerant to a tumor necrosis factor alpha (TNF-alpha) blocker or immunomodulator; or had an inadequate response with, were intolerant to, or demonstrated dependence on corticosteroids. COMPENDIAL APPROVED OFF-LABELED USES: None COVERAGE CRITERIA: INITIAL AUTHORIZATION DIAGNOSIS: Documented diagnosis of moderately to severely active ulcerative colitis (UC), OR moderately to severely active Crohn disease (CD) REQUIRED MEDICAL INFORMATION: A. CROHNS DISEASE AND ULCERATIVE COLITIS: 1. A negative tuberculin skin test (TST), or CDC-recommended equivalent, for latent tuberculosis within the last 12 months, OR No evidence of active, severe infections, significant localized infections, or sepsis as documented by a recent evaluation and screening (within the past 30 days) 2. -
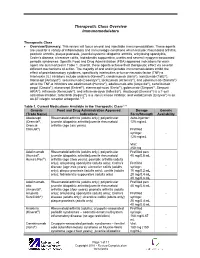
Therapeutic Class Overview Immunomodulators
Therapeutic Class Overview Immunomodulators Therapeutic Class Overview/Summary: This review will focus on oral and injectable immunomodulators. These agents are used for a variety of inflammatory and immunologic conditions which include: rheumatoid arthritis, psoriatic arthritis, plaque psoriasis, juvenile/systemic idiopathic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, hidradenitis suppurativa, uveitis and several cryopyrin-associated periodic syndromes. Specific Food and Drug Administration (FDA)-approved indications for each agent are summarized in Table 1. Overall, these agents achieve their therapeutic effect via several different mechanisms of action. The majority of oral and injectable immunomodulators inhibit the effect of proinflammatory cytokines, specifically interleukins or tumor necrosis factor (TNF)-α. Interleukin (IL) inhibitors include anakinra (Kineret®), canakinumab (Ilaris®), ixekizumab (Taltz®), rilonacept (Arcalyst®), secukinumab (Cosentyx®), tocilizumab (Actemra®), and ustekinumab (Stelara®) while the TNF-α inhibitors are adalimumab (Humira®), adalimumab-atto (Amjevita®), certolizumab pegol (Cimzia®), etanercept (Enbrel®), etanercept-szzs (Erelzi®), golimumab (Simponi®, Simponi ARIA®), infliximab (Remicade®), and infliximab-dyyb (Inflectra®). Abatacept (Orencia®) is a T-cell activation inhibitor, tofacitinib (Xeljanz®) is a Janus kinase inhibitor, and vedolizumab (Entyvio®) is an α4-β7 integrin receptor antagonist.1-19 Table 1. Current Medications Available in the Therapeutic Class1-20 -

Vedolizumab Use After Failure of TNF-Α Antagonists in Children And
Schneider et al. BMC Gastroenterology (2018) 18:140 https://doi.org/10.1186/s12876-018-0868-x RESEARCH ARTICLE Open Access Vedolizumab use after failure of TNF-α antagonists in children and adolescents with inflammatory bowel disease Anna-Maria Schneider1, Daniel Weghuber1, Benjamin Hetzer2*, Andreas Entenmann2, Thomas Müller2, Georg Zimmermann3,4, Sebastian Schütz3, Wolf-Dietrich Huber5 and Judith Pichler5 Abstract Background: Vedolizumab is safe and effective in adult patients with Crohn’s disease (CD) and ulcerative colitis (UC); however, data in children with inflammatory bowel disease (IBD) are scarce. Therefore, we evaluated vedolizumab use in a cohort of Austrian paediatric patients with IBD. Methods: Twelve patients (7 female; 7 CD; 5 UC), aged 8–17 years (median, 15 years), with severe IBD who received vedolizumab after tumour necrosis factor α antagonist treatment were retrospectively analysed. Clinical activity scores, relevant laboratory parameters, and auxological measures were obtained at infusion visits. Results: In the CD group, 1/7 patient discontinued therapy due to a severe systemic allergic reaction; 1/7 and 2/7 patients achieved complete and partial response, respectively, at week 14; and 3/7 patients discontinued therapy due to a primary non-response or loss of response. In the UC group, complete clinical remission was achieved at weeks 2, 6, and 14 in 2/5, 1/5 and 1/5 patients respectively; partial response was observed in one patient at week 2. CD activity scores did not significantly change from baseline to week 38 (median 47.5 vs. 40 points, p = 1,0), while median UC activity scores changed from 70 to 5 points (p < 0,001). -

INN Working Document 05.179 Update 2011
INN Working Document 05.179 Update 2011 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) INN Working Document 05.179 Distr.: GENERAL ENGLISH ONLY 2011 International Nonproprietary Names (INN) for biological and biotechnological substances (a review) Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Essential Medicines and Pharmaceutical Policies (EMP) International Nonproprietary Names (INN) for biological and biotechnological substances (a review) © World Health Organization 2011 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form/en/index.html). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

125476Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 125476Orig1s000 OTHER REVIEW(S) PMR/PMC Development Template This template should be completed by the PMR/PMC Development Coordinator and included for each PMR/PMC in the Action Package. NDA/BLA # BLA 125476 Product Name: Vedolizumab PMR/PMC Description: Conduct a juvenile animal toxicology study of 3 months duration in an appropriate species before initiation of the pediatric trials in patients 5 to 17 years of age.. PMR/PMC Schedule Milestones: Final Protocol Submission: February 2015 Study/Trial Completion: August 2015 Final Report Submission: February 2016 Other: None 1. During application review, explain why this issue is appropriate for a PMR/PMC instead of a pre-approval requirement. Check type below and describe. Unmet need Life-threatening condition Long-term data needed Only feasible to conduct post-approval Prior clinical experience indicates safety Small subpopulation affected Theoretical concern Other The adult studies are completed and ready for approval. 2. Describe the particular review issue and the goal of the study/clinical trial. If the study/clinical trial is a FDAAA PMR, describe the risk. If the FDAAA PMR is created post-approval, describe the “new safety information.” PMR/PMC Development Template Last Updated 5/20/2014 Page 1 of 4 Reference ID: 3509583 (b) To support the safety of vedolizumab in pediatric patients aged 5 to (4) years. The age of the monkeys used in 13-week and 26-week toxicology studies do not support the pediatric age (b) group of 5- (4) years. In the Pre and Post Natal Development (PPND) study in monkeys, adequate exposure and target saturation were not achieved in infants on postpartum (pp) days beyond 28 days. -

Benlysta® (Belimumab) Prior Authorization With
Benlysta® (belimumab) Prior Authorization with Quantity Limit Program Summary Benlysta® (belimumab) Prior Authorization with Quantity Limit TARGET AGENT Benlysta® (belimumab) Brand (generic) GPI Multisource Quantity Limit Code Benlysta (belimumab) 120 mg vial 99422015002120 M, N, O, or Y N/A 400 mg vial 99422015002140 M, N, O, or Y N/A 200 mg/mL autoinjector 9942201500D520 M, N, O, or Y 4 prefilled autoinjectors/ 28 days 200 mg/mL prefilled 9942201500E520 M, N, O, or Y 4 prefilled syringes/ 28 days syringe PRIOR AUTHORIZATION CRITERIA FOR APPROVAL Initial Evaluation Target Agent will be approved when ALL of the following are met: 1. ONE of the following: A. Information has been provided indicating that the patient is currently being treated with the requested agent within the past 90 days OR B. The prescriber states that the patient has been treated with the requested agent within the past 90 days AND is at risk if therapy is changed OR C. The patient has a diagnosis of active systemic lupus erythematosus (SLE) disease AND ALL of the following: i. The patient has a history of positive antinuclear antibody (ANA) results AND ii. The patient has a SLE additive diagnostic criteria score >10 AND iii. ONE of the following: a. BOTH of the following: a. The patient has tried and had an inadequate response to TWO of the following: corticosteroids, hydroxychloroquine, and/or immunosuppressives (i.e., azathioprine, methotrexate, oral cyclophosphamide, mycophenolate) AND b. The patient is currently being treated with at least ONE of the following: corticosteroids, hydroxychloroquine, and/or immunosuppressives (i.e., azathioprine, methotrexate, oral cyclophosphamide, mycophenolate) OR b. -

The Emerging Role of Vedolizumab in the Treatment of Ulcerative Colitis
Review: Clinical Trial Outcomes The emerging role of vedolizumab in the treatment of ulcerative colitis Clin. Invest. (2012) 2(12), 1201–1212 Ulcerative colitis is a chronic inflammatory disorder of unknown etiology. Mahmoud H Mosli1,2 Despite current treatments that include aminosalicylates, corticosteroids, & Brian G Feagan*1 antimetabolites and TNF antagonists, many patients fail to respond to 1Department of Medicine, University of Western conventional medical management and undergo colectomy. Thus, new Ontario, Robarts Clinical Trials, Robarts Research Institute, 100 Perth Dr., London, ON N6A 5K8, approaches to treatment are needed. This review discusses the emerging role Canada of vedolizumab, a humanized monoclonal antibody that selectively blocks 2Department of Medicine, King Abdulaziz lymphocyte trafficking to the gut, for the treatment of ulcerative colitis. University, Jeddah, Saudi Arabia *Author for correspondence: E-mail: [email protected] Key words: a4b7 integrin • MAdCAM-1 • therapy • ulcerative colitis • vedolizumab The idiopathic inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), are characterized by chronic intestinal inflammation that is thought to result from a pathological interaction between the immune system and gut flora[1] . CD typically causes transmural inflammation of any part of the GI tract, which may result in the complications of strictures and fistulas. In contrast, UC is characterized by superficial inflammation with a variable degree of severity [2,3]. In distinction to CD, where involvement is segmental, inflammation in UC is continuous from the anal verge and is usually restricted to the lamina propria and epithelium of the colon [1,4,5]. Typical symptoms of both conditions include bloody diarrhea, abdominal cramps and fatigue. -

Safety of Dual Biological Therapy in Crohn's Disease
Case report BMJ Open Gastroenterol: first published as 10.1136/bmjgast-2018-000243 on 9 November 2018. Downloaded from Safety of dual biological therapy in Crohn’s disease: a case series of vedolizumab in combination with other biologics Eric J Mao,1 Sara Lewin,2 Jonathan P Terdiman,2 Kendall Beck2 To cite: Mao EJ, Lewin S, ABSTRACT with mesalamine preparations, 6-mercapto- Terdiman JP, et al. Safety Uncertainty exists regarding safety and efficacy of dual purine (6-MP) monotherapy, subcutaneous of dual biological therapy biological therapy (DBT) in inflammatory bowel disease. methotrexate monotherapy and infliximab. in Crohn’s disease: a case We present four cases of DBT in Crohn’s disease. series of vedolizumab in He remained steroid dependent despite these Three patients had refractory disease non-responsive combination with other therapies. He was able to achieve a steroid-free to biological monotherapy or combination therapy biologics. BMJ Open Gastro clinical remission in his CD with adalimumab with immunomodulators. One patient had concomitant 2018;5:e000243. doi:10.1136/ monotherapy. His AS was quiescent with adal- bmjgast-2018-000243 ankylosing spondylitis. DBT was implemented by combining vedolizumab with an anti tumour necrosis imumab as well. Unfortunately, he had several lapses in adalimumab therapy leading to drug Received 3 September 2018 antibody or with ustekinumab. DBT was well-tolerated, Revised 3 October 2018 though two patients did experience self-limited infections. antibody formation and secondary loss of Protected by copyright. Accepted 16 October 2018 The efficacy of DBT remains unproven but it appears response. He was subsequently started on vedol- promising as three of the four patients achieved clinical izumab 300 mg every 8 weeks and azathioprine remission.