CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Accused Persons Arrested in Thrissur City District from 17.01.2021 to 23.01.2021
Accused Persons arrested in Thrissur City district from 17.01.2021 to 23.01.2021 Name of Name of the Name of the Place at Date & Arresting Court at Sl. Name of the Age & Cr. No & Sec Police father of Address of Accused which Time of Officer, which No. Accused Sex of Law Station Accused Arrested Arrest Rank & accused Designation produced 1 2 3 4 5 6 7 8 9 10 11 MARUTHOOR KARTHIAYA Thrissur HOUSE, NI TEMPLE 23-01-2021 RAMACHA 33, 80/2021 U/s West BYJU K.C. SI BAILED BY 1 RAGHU KARTHIAYANI ROAD at 22:05 NDRAN Male 151 CrPC (Thrissur OF POLICE POLICE TEMPLE, AYYANTHO Hrs City) AYYANTHOLE LE Thrissur ODAYIL 23-01-2021 122/2021 U/s RADHAKRI 36, NR KALYAN East ANUDAS .K, BAILED BY 2 RAKESH (H),KUTTUMUKKU at 23:00 279 IPC & 185 SHNAN Male JEWELLERS (Thrissur SI OF POLICE POLICE , THRISSUR Hrs MV ACT City) Koothumakkal 23-01-2021 71/2021 U/s Peramangal 48, BAILED BY 3 Sreekumar Appu House, Varadiyam at 20:15 118(e) of KP am (Thrissur Sreejith S I Male POLICE Peringottukara Hrs Act City) CHOONDAKARA Thrissur N (H), NEAR 23-01-2021 26, 121/2021 U/s East ANUDAS .K, BAILED BY 4 THOMAS PAULSON THOTTAPPADY, SAPNA at 20:35 Male 279, 283 IPC (Thrissur SI OF POLICE POLICE ANCHERY, THEATRE Hrs City) THRISSUR ERATH (H), Thrissur 23-01-2021 119/2021 U/s 27, VALARKAVU, BTR ITC East ANUDAS .K, BAILED BY 5 SANOOP SUNIL at 19:00 279 IPC & 185 Male NAGAR, JUNCTION (Thrissur SI OF POLICE POLICE Hrs MV ACT KURIACHIRA City) KEEDAM KUNNATH(H)THI 51/2021 U/s PAZHAYA NIZAMUDDI 23-01-2021 GOPALAKR RAMAKRIS 39, RUVADI,PUDUKK PAZHAYAN 279 IPC & NNUR N J, BAILED BY 6 at -

Kerala Industrial Infrastructure
SECTION 2.00 – GENERAL CONDITIONS OF CONTRACT 15 GENERAL CONDITIONS OF CONTRACT 2.1.0 General conditions The system should be complete functional unit in terms of hardware and software to demonstrate the intended specifications and applications to be supplied with all necessary ancillaries. The quote should be for supply, installation, commissioning and successful working demonstration of the instrument at the premises of M/s. Oushadhi Ltd, Kuttanellur, Thrissur, Kerala. Specifications should be confirmed from the technical Brochure and website of the manufacturer. Good Service and Technical support are essential. Please provide testimonials form three reputed customers. Training: 2 levels – 5 days during installation in our lab and subsequently at supplier’s application lab for 2 persons at suppliers’ cost, three times in a duration of 3 years. A compliance statement of the specifications should be provided along with the quote before placing order. The list of installations and users of complete system in India & abroad should be provided for reference. The company must have at least five working installations of complete system (HPTLC) globally. User list for this period, current contact details of users, Supply Orders and certificates of successful completion issued by the clients must be enclosed. Installation, calibration, standardization and commissioning shall be the responsibility of the vendor. Pre-installation requirements, including requirement for water/power supply, should be enclosed along with the tender. Comprehensive maintenance contract (C.M.C )for 3 years with preventive maintenance kits 2.1.1 DEFINITION OF TERMS In construing these General Conditions of Contract and the annexed Technical Specifications and Commercial Terms, the following words shall have the meanings herein assigned to them unless there is something in the subject or context inconsistent with such construction. -

The Heart of Kerala!
Welcome to the Heart of Kerala! http://www.neelambari.co.in w: +91 9400 525150 [email protected] f: http://www.facebook.com/NeelambariKerala Overview Neelambari is a luxurious resort on the banks of Karuvannur puzha (river). It is constructed in authentic Kerala style and evokes grandeur and tradition. The central building consists of a classical performance arena (Koothambalam) and a traditional courtyard (Nalukettu). The cottages are luxurious with their own private balconies, spacious and clean bathrooms and well appointed bedrooms (each unit has a space of more than 75 sqm). Neelambari is situated in a very serene atmosphere right on the bank of a river, in a quiet, verdant village in central Kerala. There are several natural and historical attractions in the vicinity. Despite its rural charm, the facility is well connected, being less than an hour drive from Cochin International Airport. It is also easily accessible by rail and road and the nearest city is Thrissur, just 13 kms away. The facility offers authentic Ayurveda treatment, Yoga lessons, nature and village tourism, kayak and traditional boat trips in the river as well as traditional cultural performances in its Koothambalam. http://www.neelambari.co.in w: +91 9400 525150 [email protected] f: http://www.facebook.com/NeelambariKerala Our location Neelambari is located in Arattupuzha, a serene little village in the outskirts of Thrissur City. Thrissur has a rightful claim as the cultural capital of Kerala for more reasons than one. A host of prestigious institutions that assiduously preserve and nurture the cultural traditions of Kerala such as the Kerala Sangeetha Nataka Academy, Kerala Sahitya Academy, Kerala Lalitha Kala Academy, Kerala Kalamandalam, Unnayi Warrier Kalanilayam are located in Thrissur. -
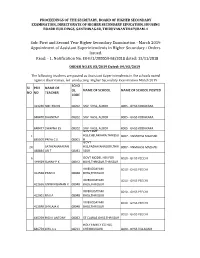
Sub: First and Second Year Higher Secondary Examination - March 2019 Appointment of Assistant Superintendents in Higher Secondary - Orders Issued
PROCEEDINGS OF THE SECRETARY, BOARD OF HIGHER SECONDARY EXAMINATION, DIRECTORATE OF HIGHER SECONDARY EDUCATION, HOUSING BOARD BUILDINGS, SANTHINAGAR, THIRUVANANTHAPURAM-1 Sub: First and Second Year Higher Secondary Examination - March 2019 Appointment of Assistant Superintendents in Higher Secondary - Orders Issued. Read: - 1. Notification No. EX-II/1/20000/HSE/2018 dated: 13/11/2018 ORDER NO.EX 03/2019 Dated: 04/03/2019 The following teachers are posted as Assistant Superintendents in the schools noted against their names, for conducting Higher Secondary Examination March 2019 SCHO SI PEN NAME OF OL NAME OF SCHOOL NAME OF SCHOOL POSTED NO NO TEACHER CODE 323246 SMITHA KN 08202 SNV VHSS, ALOOR 8005 - GHSS KODAKARA 848470 DHANYA P 08202 SNV VHSS, ALOOR 8005 - GHSS KODAKARA 848477 SWAPNA ES 08202 SNV VHSS, ALOOR 8005 - GHSS KODAKARA GOVT SMT 1 HSS,CHELAKKARA,THRISSU 8007 - VNMGHSS MACHAD 835500 PRIYA C S 08001 R GOVT 24 SATHYANARAYAN HSS,PAZHAYANNOOR,THRI 8007 - VNMGHSS MACHAD 448844 AN T 08041 SSUR 6 GOVT MODEL HSS FOR 8010 - GHSS PEECHI 449429 SUNNY P K 08012 BOYS,THRISSUR,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412558 PRAN K 08048 BHSS,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412636 UNNIKRISHNAN K 08048 BHSS,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412601 RAJI A 08048 BHSS,THRISSUR VIVEKODAYAM 8010 - GHSS PEECHI 412598 SHYLAJA K 08048 BHSS,THRISSUR 8010 - GHSS PEECHI 645769 RIGI K ANTONY 08052 ST CLARAS GHSS,THRISSUR HOLY FAMILY CG HSS, 846759 LIRIL C L 08213 CHEMBUKAVU 8020 - GHSS VILLADAM SCHO SI PEN NAME OF OL NAME OF SCHOOL NAME OF SCHOOL POSTED NO NO TEACHER CODE ST AUGUSTINE HSS 846381 JINCY P FRANCIS 08215 KUTTANELLUR, THRISSUR 8020 - GHSS VILLADAM DENNIS XAVIER ST AUGUSTINE HSS 319862 AKKARA 08215 KUTTANELLUR, THRISSUR 8020 - GHSS VILLADAM 324459 SREEJISH P K 08206 CHENTHRA PINNI HSS 8022 - GHSS CHAVAKKAD ST. -

Ss of the Sector Product (S) Enterprise # 1 Haritham Arts&Crafts Traditional Handicraft Thrikaipetta (Po), Nellimalam, Wayanad T-9495240567
NAME & ADDRESS OF THE SECTOR PRODUCT (S) ENTERPRISE # 1 HARITHAM ARTS&CRAFTS TRADITIONAL HANDICRAFT THRIKAIPETTA (PO), NELLIMALAM, WAYANAD T-9495240567 2 ABHIRAMI BAMBOO UNIT TRADITIONAL BAMBOO PAZHUPPATHUR P.O PRODUCTS THEKKINKANDY WAYANAD-673592 T-9539595919 3 AISWARYA NUTIMIX FOOD NUTRIMIX NENMENIKKUNNU P.O PROCESSING SULTHAN BETHERY WAYANAD- 673595 T-9747075572 4 SWATHY CURRY POWDER FOOD CURRY POWDER THAVANI PROCESSING NENMENI P.O KOLIYADI SULTHAN BETHERY WAYANAD MOB: 9605974980 5 VALSALYAM NUTRIMIX FOOD CURRY POWDER/ NENMENI.P.O PROCESSING NUTRIMIX MADAKARA SULTHAN BETHERI WAYANAD MOB: 9656051316 [email protected] OM 6 AISWARYA NUTIMIX FOOD NUTRIMIX NOOLPUZHA PANJAYAT PROCESSING NENMENI KUNNU P.O WAYANAD- 673595 MOB: 9744540040 7 JWALA NUTRIMIX FOOD NUTRIMIX KIDAGANAD P O PROCESSING VADAKKANAD SULTHAN BETHERY WAYANAD MOB: 9961137711 8 JEEVANDHARA NEUTRIMIX FOOD NUTRIMIX KIDAGANAD P.O PROCESSING VADAKKANAD WAYANAD MOB: 9744667166 9 JEEVANDHARA NEUTRIMIX FOOD NUTRIMIX KIDAGANAD P.O PROCESSING VADAKKANAD WAYANAD MOB: 9747574461 10 ATHIRA FOOD PRODUCTS FOOD FOOD PRODUCT K21, USHAS PROCESSING KANIYARAM MANANTHAVADY WAYANAD PH: 04935240185 , 9446648051 11 AYSHA CHEMICALS CHEMICALS DETERGENTS POOTHICAUD POOMALA P O WAYANAD PH: 04936222166 12 KOCHIKUNNEL FOOD FOOD BAKERY PRODUCTS KINFRA PARK PROCESSING PRODUCTS CHUNDEL KALPETTA WAYANAD PH: 04936202707 , 9447042677 KOCHIKUNNELFOODPRODUCTS@G MAIL.COM 13 NAS BAGS OTHERS SHOPPER BAGS PUZHAMUDI P.O KALPETTA WAYANAD T-04936204669 , 9567455570 14 KENZ GARMENTS, GARMENTS READYMADE DWARAKA, -

Ac Name Ac Addr1 Ac Addr2 Ac Addr3 Sobha S Pai Sports Land, Old Thirumala, Alleppey Pin; 688 011 Beena Jose W/O Jose C P Chutti
AC_NAME AC_ADDR1 AC_ADDR2 AC_ADDR3 SOBHA S PAI SPORTS LAND, OLD THIRUMALA, ALLEPPEY PIN; 688 011 BEENA JOSE W/O JOSE C P CHUTTIKANNAN HOUSE KODIKUTHUMALA DEEPA MARY MATHEWS CHEERAN HOUSE DOCTORS LANE U C COLLEGE P O ALUVA IYPE M J MANJOORAN HOUSE ASOKAPURAM ALUVA PREETHA KRISHNAKUMAR SASTHANGAL WARRIAM DESOM P O ALUVA REMANI S NAIR MANCERRY HOUSE KOOVAPPADY P O PERUMBAVOOR SIGI ABRAHAM KOOTTANCHERY ERUMATHALA PO KEEZHUMAD ALUVA DEVASSY T D THALIYATH HOUSE KUMBIDY POOVATHUSSERY P O THRISSUR DISTRICT I K UNNIKRISHNAN IYYEDATH HOUSE POOVATHUSSERY P O JASVIN JOHN THEKKINIYAN HOUSE POOVATHUSSERRY 680741 KUTTAPPAN NAIR V VELUKKATHARA HOUSE KALLUR ANNAMANADA P O NOUSHAD.V.M. AISHA MANZIL ANNAMANADA.P.O. MALLIKA C N . REXY RODRIGUES KOLLAMVALIYAKAM KADUKUTTY PO CALAKUDY SHOJI DAVIS WILSON STUDIO MALA ST JOSEPH CATECHISM UNIT . PAZHOOKARA,ANNALLOOR (PO) THRISSUR DISTRICT RAZAK M A MANOLODY HOUSE VAYALKARA KUNNUKARA HAMZA K M K M HOUSE POOTHAPARA,AZHIKODE GEORGE Z BERNARD ST.JOSEPH COLLEGE OF COMMERCE,73, BRIGADE ROAD,BANGALORE LILLY RAJU C-PH2 MANTRI SPLENDOR, HENNUR MAIN ROAD, GEDDALAHALLI,BANGALORE. NEETHU P CHEMMANNUR JEWELERS UNITY BUILDING J C ROAD BANGALORE SHAJU C MATHEW CHEMMANNUR JEWLLERS J C ROAD, UNITY BUILDING BANGALORE SHANOJ E U CHEMMANNUR JEWELLERS LTD J C ROAD BANGALORE V.VARDHAN L.R.NAGAR, D NO 349, 12TH CROSS, BANGALORE 560 047. NITHISH PAUL 301-B,SUMER CASTLE, CASTLE MILL COMPOUND,. THANE (W)-400 602 PRIYA MALHOTRA 305, GANGA ESTATE CHEMBUR, MUMBAI-71 RAMYA BHASKARAN PILLAI 34,GANGA SHREE NARAYANA GURU- CHS;LOKHANDE MARG,CHEMBUR, -

1 TRICHUR 940 B Urban RBO 1 Thrissur 9654 2
ANNEXURE-A LIST OF BRANCHES UNDER RBO-I,THRISSUR BRANCH U/SU/RURAL Area(in SL. NO BRANCH NAME CODE CATEGORY REGION SQFT) 1 TRICHUR 940 B Urban RBO 1 Thrissur 9654 2 TRICHUR TOWN 8679 B Urban RBO 1 Thrissur 5438 3 PEECHI 2255 B Semi Urban RBO 1 Thrissur 2700 4 CHERPU 8606 B Rural RBO 1 Thrissur 2970 5 KURIACHIRA 8636 B Rural RBO 1 Thrissur 2595 6 VALLACHIRA 8684 B Semi Urban RBO 1 Thrissur 1670 7 VILANGAN 8693 B Rural RBO 1 Thrissur 3356 8 EAST FORT (TRICHUR) 9121 B Urban RBO 1 Thrissur 3356 Urban 9 POLICE ACADEMY TRICHUR 10566 B RBO 1 Thrissur 800 10 KUTTANELLUR 11930 B Urban RBO 1 Thrissur 2500 Urban RBO 1 Thrissur 11 SHAKTAN NAGAR TRICHUR 12892 B 1850 12 NRI TRICHUR 14466 B Urban RBO 1 Thrissur 1968 13 KUHAS TRICHUR 14682 B Rural RBO 1 Thrissur 3000 14 POONKUNNAM 16080 B Urban RBO 1 Thrissur 1995 15 SPBB THRISSUR 16085 B Urban RBO 1 Thrissur 2604 16 MANNUTHY 16494 B Urban RBO 1 Thrissur 1887 17 OLARI 16658 B Urban RBO 1 Thrissur 2998 18 PERINGAVU 18115 B Urban RBO 1 Thrissur 2947 19 KANJANI TOWN 18877 B Semi Urban RBO 1 Thrissur 1700 PUNKUNNAM RAILWAY Urban RBO 1 Thrissur 20 STATION, THRISSUR 21787 B 1650 21 THRISSUR- CIVIL STATION 70164 B Urban RBO 1 Thrissur 2165 22 THRISSUR- ROUND SOUTH 70165 B Urban RBO 1 Thrissur 3183 23 KURKANCHERRY 70174 B Urban RBO 1 Thrissur 2498 24 URAKOM 70175 B Semi Urban RBO 1 Thrissur 3300 25 CHERUR 70207 B Semi Urban RBO 1 Thrissur 3750 26 OLLUKARA 70210 B Urban RBO 1 Thrissur 4056 27 THRISSUR ADB 70253 B Semi Urban RBO 1 Thrissur 3400 28 OLLUR 70266 B Urban RBO 1 Thrissur 3416 29 THALORE 70470 B Semi -

Crisis Management Plan Thrissur Pooram 2018
T H R I S S U R P O O R A M Sree Vadakkunnatha Temple Thiruvambady Temple Paramekkavu Bhagathy Temple Chekbukkavu Bhagavathy Temple Kanimangalam SasthaTemple Panamukkumpally Sastha Temple Paramekkavu Bhagathy Temple Karamukku Bhagavathy Temple Laloor Bhagavathy Temple Choorakkottukavu Bhagavathy Temple Paramekkavu Bhagathy Temple Ayyanthole Bhagavathy Temple Neithalakkavu Bhagavathy Temple : Thrissur Pooram 2018 : : CHAPTER – I : [ INTRODUCTION 1.1 PURPOSE The purpose of the Crisis Management Plan for Thrissur Pooram is to set out actions to be taken by the in the event of any crisis or emergency occurring in connection with Thrissur Pooram. The Crisis Management Plan is designed to assist Crowd Management and Emergency Operations and creation of a system for protection of life and property in the event of a natural, manmade or hybrid hazard requiring emergency activation. The Crisis Management Plan provides guidance for all line departments in order to minimize threats to life and property. 1.2 SCOPE Crisis Management Plan covers all phases of crisis management right from mitigation, preparedness, emergency response, relief to recovery. The plan discusses roles and responsibilities of each stakeholder and should be used as a guide by all the concerned line departments to prepare their respective department to play these critical roles and responsibilities. 1.3 OBJECTIVE To protect the life of people who have assembled for the event and to avoid confusion among major stakeholders during emergency and to develop a basic structure for time sensitive, safe, secure, orderly and efficiently handling the crisis. Crisis Management Plan Page No: 1 : Thrissur Pooram 2018 : Aerial VIEW of THEKINKAD MAIDAN Page No: 2 Crisis Management Plan : Thrissur Pooram 2018 : CHAPTER – 2 [THRISSUR POORAM – POORAM OF CULTURAL CAPITAL 2.1 HISTORY & RITUALS OF THRISSUR POORAM Life in Kerala is punctuated by the annual festivals dedicated to village deities. -

Supply of Medicinal Raw Materials the Achilles Heel of Today’S Manufacturing Sector for Ayurvedic Drugs in Kerala
asian medicine 9 (2014) 206–235 brill.com/asme Supply of Medicinal Raw Materials The Achilles Heel of Today’s Manufacturing Sector for Ayurvedic Drugs in Kerala Lucie Dejouhanet University of the French Antilles, Laboratory AIHP-EODE, EA 929 [email protected] Abstract The growth of the manufacturing sector in Ayurveda seems to be slowing down in the Indian state of Kerala as the prices of raw materials have increased inordinately, chal- lenging the sustainability of ayurvedic drug production. Several reasons are responsible; in particular, the dwindling availability of plants, the excessive complexity of supply chains, the growing distances between plant sources and manufacturing units, and the control over the medicinal plant market by powerful middlemen. Substitution, adul- teration, and quality control have become sensitive issues and may eventually damage the reputation of the ayurvedic drug sector in a context of high competition between manufacturers. Cultivating medicinal plants is considered to be the main solution for solving the supply crisis, but manufacturers’ demands are often too constraining for cultivators and this may not be such a viable alternative in a context of continuous modernisation efforts for production efficiency. Keywords raw materials – supply chain – cultivation – quality control – traceability Introduction The huge development of the Ayurveda pharmaceutical sector in Kerala since the 1980s is nowadays challenged by the sector’s difficulty to get a steady sup- ply of raw materials that is necessary for pursuing smooth production. In 2006, 91% of the herbal raw material used in Ayurveda came from the wild. Of the material sourced by the manufacturers from Kerala, 43% grew in forests, 16% © koninklijke brill nv, leiden, 2�15 | doi 10.1163/15734218-12341293Downloaded from Brill.com10/01/2021 07:09:06AM via free access Supply of Medicinal Raw Materials 207 in non-forested areas, 18% came from both ecosystems, and 14% from out- side Kerala, mostly North India. -
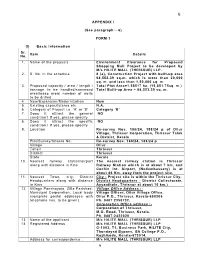
FORM 1 (I) Basic Information Sr. No. Item Details 1. Name of the Project/S
6 APPENDIX I (See paragraph – 6) FORM 1 (I) Basic Information Sr. Item Details No. 1. Name of the project/s Environment Clearance for Proposed Shopping Mall Project to be developed by M/s HILITE MALL (THRISSUR) LLP. 2. S. No. in the schedule 8 (a), Construction Project with built-up area 68,553.39 sq.m. which is more than 20,000 sq. m. and less than 1,50,000 sq. m. 3. Proposed capacity / area / length / Total Plot Area=1.58517 ha. (15,851.75sq. m.) tonnage to be handled/command Total Built-up Area = 68,553.39 sq. m. area/lease area/ number of wells to be drilled 4. New/Expansion/Modernization New 5. Existing capacity/area etc., N.A. 6. Category of Project i.e. ‘A’ or ‘B’ Category ‘B’ 7. Does it attract the general NO condition? If yes, please specify 8. Does it attract the specific NO condition? If yes, please specify 9. Location Re-survey Nos. 188/24, 188/24 p of Ollur Village, Thrissur Corporation, Thrissur Taluk & District, Kerala Plot/Survey/Khasra No. Re-survey Nos. 188/24, 188/24 p Village Ollur Tehsil Thrissur District Thrissur State Kerala 10. Nearest railway station/airport The nearest railway station is Thrissur along with distance in Kms Railway Station which is at about 7 km. and Cochin Int. Airport, (Nedumbassery) is at about 46 Km. away from the project site. 11. Nearest Town, city, District City - Project site is within the Thrissur City. Headquarters along with distance District Headquarters - District Collectorate, in Kms Ayyanthole, Thrissur at about 10 km.) 12 Village Panchayats, Zilla Parishad, Village Office Address :- Municipal Corporation, Local body Village Officer, Ollur Village Office, (complete postal addresses with Ollur P.O., Thrissur, Kerala-680306 telephone nos. -

State of Kerala & Mahe District of UT of Puducherry In
Notice for appointment of Regular / Rural Retail Outlet Dealerships - State of Kerala & Mahe District of UT of Puducherry Indian Oil Corporation proposes to appoint Retail Outlet dealers in the State of Kerala & Mahe District of UT of Puducherry, as per following details: Minimum Dimension Finance to be Fixed fee/Minimum Bid Estimated monthly Type of Mode of Security deposit Sl. Name of location Revenue District Type of RO Category (in M.)/Area of the site arranged amont sales Potential # site* Selection (Rs in lakhs) No. ( in Sq.M.)* by the Applicant (Rs. In lakhs) 1 2 3 4 5 6 7 8 9a 9b 10 11 12 Regular/Rural MS + HSD in Kls SC, SC Cc1, CC/DC/CFS Frontage Depth Area Estimated Estimated fund Draw of Lots/Bidding SC CC2,SC PH, working capital required for ST, ST CC1, ST requirement for development of CC2, ST PH, operation of infrastructure OBC, OBC Cc1, RO at RO OBC CC2, OBC PH, OPEN, OPEN CC1, OPEN CC2, OPEN PH 1 Punnumoodu Alappuzha Regular 160 SC CFS 25 25 625 0 0 Draw of Lots 0 3 2 Mararikulam to Thaikkal Beach on SH 66 Alappuzha Regular 130 SC CFS 30 30 900 0 0 Draw of Lots 0 3 3 Kavalam - Kidangara Road Alappuzha Rural 100 SC CFS 20 20 400 0 0 Draw of Lots 0 2 4 Angamaly Jn - Adlux International (NH - LHS) Ernakulam Regular 150 SC CFS 35 45 1575 0 0 Draw of Lots 0 3 5 Kunnumpuram Jn, Kakkanad to Thrikkakara Ernakulam Regular 160 SC CFS 25 25 625 0 0 Draw of Lots 0 3 on Kunnumpuram NGO Quarters Road 6 Fort Kochi to Mattancherry Ernakulam Regular 160 SC CFS 25 25 625 0 0 Draw of Lots 0 3 7 Puthencruz to Kolenchery Ernakulam Regular -
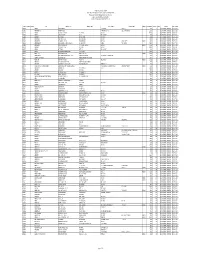
CSBL Unpaid Dividend, Refund Consolidated As on 22.09.2015.Xlsx
The Catholic Syrian Bank Limited Regd. Office, "CSB Bhavan", St. Mary's College Road, Thrissur 680020 Phone: 0487 -2333020, 6451640, eMail: [email protected] List of Unpaid Dividend as on 22.09.2015 (Dividend for the periods 2007-08 to 2013-14) FOLIO / DEMAT ID INITLS NAME ADDRESS LINE 1 ADDRESS LINE 2 ADDRESS LINE 3 ADDRESS LINE 4 PINCOD DIV.AMOUNT DWNO MICR PERIOD IEPF. TR. DATE A00350 ANTONY PALLANS HOUSE KURIACHARA TRICHUR, 30.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00385 ANNAMMA P X AKKARA HOUSE PANAMKUTTICHIRA OLLUR, TRICHUR DIST 150.00 5 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00398 ANTONY KUTTENCHERY HOUSE HIGH ROAD TRICHUR 1020.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00406 ANTONY KALLIATH HOUSE OLLUR TRICHUR DIST 27.00 9 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00409 ANTHONY PLOT NO 143 NEHRU NAGAR TRICHUR-6 120.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00643 ANTHAPPAN PADIKKALA HOUSE EAST FORT GATE TRICHUR 540.00 12 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00647 ANTHONY O K OLAKKENGAL HOUSE LOURDEPURAM TRICHUR - KERALA STATE. 680005 180.00 13 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00668 ANTHONISWAMI C/O INASIMUTHU MUDALIAR SONS 55 NEW STREET KARUR TAMILNADU 2100.00 14 2007-08 UNPAID DIVIDEND 25-OCT-2015 A00822 ANNA JACOB C/O J S MANAVALAN 5 V R NAGAR ADAYAR MADRAS - 600020 210.00 18 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01072 ANTHONY VI/62 PALACE VIEW EAST FORT TRICHUR 4200.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01077 ANTONY KOTTEKAD KUTTUR TRICHUR DIST 30.00 0 2007-08 UNPAID DIVIDEND 25-OCT-2015 A01103 ANTONY ELUVATHINGAL CHERUVATHERI