A New Generation of Antidepressants: an Update on the Pharmaceutical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract Book – Oral Sessions
Sunday, June 21, 2015 12:00 p.m. - 1:15 p.m. Latin American Psychopharmacology Update: INNOVATIVE TREATMENTS IN PSYCHIATRY INNOVATIVE TREATMENTS IN PSYCHIATRY Flavio Kapczinski, The University of Texas Health Science Center at Houston Overall Abstract In this panel, we will propose innovative treatments in psychiatry and recent literature findings will be summarized. The effective pharmacological treatment of psychiatric diseases and development of new therapeutic entities has been a long-standing challenge. Despite the complexity and heterogeneity of psychiatric disorders, basic and clinical research studies and technological advancements in genomics, biomarkers, and imaging have begun to elucidate the pathophysiology of etiological complexity of psychiatric diseases and to identify efficacious new agents (Tcheremissine et al. 2014). Many psychiatric illnesses are associated with neuronal atrophy, characterized by loss of synaptic connections, increase of inflammatory markers like TNF-α and DAMPS (damage-associate molecular patterns,- cell free (ccf) DNA, heat shock proteins HSP70, HSP90, and HSP60, and cytochrome C), decrease of neurotrophic factors, increase of oxidative stress, mitochondrial dysfunction and apoptosis (Fries et al., 2012; Pfaffenseller et al., 2014). Also, neurocognitive impairment and poor psychosocial functioning has been related to a psychiatric disease. Therefore, trying to discover new therapeutic targets that act in these pathways can help to develop new treatment or improve the treatment of these serious diseases. -

Metabotropic Glutamate Receptors
mGluR Metabotropic glutamate receptors mGluR (metabotropic glutamate receptor) is a type of glutamate receptor that are active through an indirect metabotropic process. They are members of thegroup C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatoryneurotransmitter. The mGluRs perform a variety of functions in the central and peripheral nervous systems: mGluRs are involved in learning, memory, anxiety, and the perception of pain. mGluRs are found in pre- and postsynaptic neurons in synapses of the hippocampus, cerebellum, and the cerebral cortex, as well as other parts of the brain and in peripheral tissues. Eight different types of mGluRs, labeled mGluR1 to mGluR8, are divided into groups I, II, and III. Receptor types are grouped based on receptor structure and physiological activity. www.MedChemExpress.com 1 mGluR Agonists, Antagonists, Inhibitors, Modulators & Activators (-)-Camphoric acid (1R,2S)-VU0155041 Cat. No.: HY-122808 Cat. No.: HY-14417A (-)-Camphoric acid is the less active enantiomer (1R,2S)-VU0155041, Cis regioisomer of VU0155041, is of Camphoric acid. Camphoric acid stimulates a partial mGluR4 agonist with an EC50 of 2.35 osteoblast differentiation and induces μM. glutamate receptor expression. Camphoric acid also significantly induced the activation of NF-κB and AP-1. Purity: ≥98.0% Purity: ≥98.0% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10 mM × 1 mL, 100 mg Size: 10 mM × 1 mL, 5 mg, 10 mg, 25 mg (2R,4R)-APDC (R)-ADX-47273 Cat. No.: HY-102091 Cat. No.: HY-13058B (2R,4R)-APDC is a selective group II metabotropic (R)-ADX-47273 is a potent mGluR5 positive glutamate receptors (mGluRs) agonist. -

RAP-MD-05 GLYX13-C-203 Protocol Amendment 3 Final-R
NCT02192099 Study ID: GLYX13‐C‐203 Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX‐13) (Extension of GLYX13‐C‐202, NCT01684163) Protocol Amendment 3 Date: 23 Nov 2016 Clinical Study Protocol Protocol Number: GLYX13-C-203 Study Drug: Rapastinel (GLYX-13) Injection (rapastinel Injection) FDA ID: 107,974 Clinicaltrials.gov: Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX-13) (Extension of GLYX13-C-202, NCT01684163) Study Phase: 2 Sponsor: Naurex, Inc, an affiliate of Allergan, plc. Date: Amendment 3 dated 23 November 2016 Replaces Amendment 2 dated 20 January 2015 This document is a confidential communication from Naurex, Inc, an affiliate of Allergan, plc.. Acceptance of this document constitutes an agreement by the recipient(s) that no information contained herein will be published or disclosed without prior written approval from Allergan, plc., except that this document may be disclosed to appropriate Institutional Review Boards (IRBs) under the condition that they are also required to maintain confidentiality. Naurex, Inc, an affiliate of Allergan, plc. Protocol GLYX13-C-203 INVESTIGATOR SIGNATURE PAGE The signature of the investigator below constitutes his/her approval of this protocol and provides the necessary assurances that this study will be conducted according to all stipulations of the protocol as specified in both the clinical and administrative sections, including all statements regarding confidentiality. This trial will be conducted in compliance with the protocol and all applicable regulatory requirements, in accordance with Good Clinical Practices (GCPs), including International Conference on Harmonization (ICH) Guidelines, and in general conformity with the most recent version of the Declaration of Helsinki. -

Summary Analgesics Dec2019
Status as of December 31, 2019 UPDATE STATUS: N = New, A = Advanced, C = Changed, S = Same (No Change), D = Discontinued Update Emerging treatments for acute and chronic pain Development Status, Route, Contact information Status Agent Description / Mechanism of Opioid Function / Target Indication / Other Comments Sponsor / Originator Status Route URL Action (Y/No) 2019 UPDATES / CONTINUING PRODUCTS FROM 2018 Small molecule, inhibition of 1% diacerein TWi Biotechnology / caspase-1, block activation of 1 (AC-203 / caspase-1 inhibitor Inherited Epidermolysis Bullosa Castle Creek Phase 2 No Topical www.twibiotech.com NLRP3 inflamasomes; reduced CCP-020) Pharmaceuticals IL-1beta and IL-18 Small molecule; topical NSAID Frontier 2 AB001 NSAID formulation (nondisclosed active Chronic low back pain Phase 2 No Topical www.frontierbiotech.com/en/products/1.html Biotechnologies ingredient) Small molecule; oral uricosuric / anti-inflammatory agent + febuxostat (xanthine oxidase Gout in patients taking urate- Uricosuric + 3 AC-201 CR inhibitor); inhibition of NLRP3 lowering therapy; Gout; TWi Biotechnology Phase 2 No Oral www.twibiotech.com/rAndD_11 xanthine oxidase inflammasome assembly, reduced Epidermolysis Bullosa Simplex (EBS) production of caspase-1 and cytokine IL-1Beta www.arraybiopharma.com/our-science/our-pipeline AK-1830 Small molecule; tropomyosin Array BioPharma / 4 TrkA Pain, inflammation Phase 1 No Oral www.asahi- A (ARRY-954) receptor kinase A (TrkA) inhibitor Asahi Kasei Pharma kasei.co.jp/asahi/en/news/2016/e160401_2.html www.neurosmedical.com/clinical-research; -

Sciatica and Chronic Pain
Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh 123 Sciatica and Chronic Pain Robert W. Baloh Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh, MD Department of Neurology University of California, Los Angeles Los Angeles, CA, USA ISBN 978-3-319-93903-2 ISBN 978-3-319-93904-9 (eBook) https://doi.org/10.1007/978-3-319-93904-9 Library of Congress Control Number: 2018952076 © Springer International Publishing AG, part of Springer Nature 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

A Human Stem Cell-Derived Test System for Agents Modifying Neuronal N
Archives of Toxicology (2021) 95:1703–1722 https://doi.org/10.1007/s00204-021-03024-0 IN VITRO SYSTEMS A human stem cell‑derived test system for agents modifying neuronal 2+ N‑methyl‑D‑aspartate‑type glutamate receptor Ca ‑signalling Stefanie Klima1,2 · Markus Brüll1 · Anna‑Sophie Spreng1,3 · Ilinca Suciu1,3 · Tjalda Falt1 · Jens C. Schwamborn4 · Tanja Waldmann1 · Christiaan Karreman1 · Marcel Leist1,5 Received: 28 October 2020 / Accepted: 4 March 2021 / Published online: 13 March 2021 © The Author(s) 2021 Abstract Methods to assess neuronal receptor functions are needed in toxicology and for drug development. Human-based test systems that allow studies on glutamate signalling are still scarce. To address this issue, we developed and characterized pluripotent stem cell (PSC)-based neural cultures capable of forming a functional network. Starting from a stably proliferating neu- roepithelial stem cell (NESC) population, we generate “mixed cortical cultures” (MCC) within 24 days. Characterization by immunocytochemistry, gene expression profling and functional tests (multi-electrode arrays) showed that MCC contain various functional neurotransmitter receptors, and in particular, the N-methyl-D-aspartate subtype of ionotropic glutamate receptors (NMDA-R). As this important receptor is found neither on conventional neural cell lines nor on most stem cell- derived neurons, we focused here on the characterization of rapid glutamate-triggered Ca2+ signalling. Changes of the intra- 2+ cellular free calcium ion concentration ([Ca ]i) were measured by fuorescent imaging as the main endpoint, and a method to evaluate and quantify signals in hundreds of cells at the same time was developed. We observed responses to glutamate in the low µM range. -

Stock Price Effects of Breakthrough Therapy Designation
NEWS & ANALYSIS SUPPLEMENTARY INFORMATION BIOBUSINESS BRIEFS In format as provided by the authors Stock price effects of breakthrough therapy designation David Hoffmann, Shane Van Dalsem and Frank S. David NATURE REVIEWS | D RU G D IS COVERY Supplementary Box 1 | Methods and data 1. Methods 1a. Sample definition and data collection Event data collection The website Friends of Cancer Research (http://www.focr.com/) lists all publicly available Breakthrough Therapy Designations (BTD), which were downloaded with a cut-off on 06/30/18. For partnered products, we treated each partner as if it had independently received the BTD, and thus generated an entry for each co-developing company. For each BTD, we identified the original press release and excluded companies that did not disclose the exact BTD announcement date. Further, we excluded companies that are not publicly traded on a US stock exchange, and those that had incomplete stock price data in the event period (as of 08/24/18). We also excluded BTDs that coincided with major corporate press releases (e.g., quarterly results or clinical trial news). In cases where the same company announced several BTDs on the same date, we retained only one for analysis. This yielded 146 BTDs (102 commercial, 44 pre-commercial). Subsequently, we identified three outliers and excluded them from the final CAAR analysis (see later section). 1 Stock price collection The historical closing stock prices (unadjusted) for the range of -111 days to +90 days around the disclosure of the BTD events were downloaded from https://finance.yahoo.com/. Historical stock prices from delisted companies were downloaded from https://amigobulls.com or https://barchart.com. -

Poster Session III, December 6, 2017
Neuropsychopharmacology (2017) 42, S476–S652 © 2017 American College of Neuropsychopharmacology. All rights reserved 0893-133X/17 www.neuropsychopharmacology.org Poster Session III a high-resolution research tomograph (HRRT), and struc- Palm Springs, California, December 3–7, 2017 tural (T1) MRI using a 3-Tesla scanner. PET data analyses were carried out using the validated 2-tissue compartment Sponsorship Statement: Publication of this supplement is model to determine the total volume of distribution (VT) of sponsored by the ACNP. [18F]FEPPA. Individual contributor disclosures may be found within the Results: Results show significant inverse associations (con- abstracts. Part 1: All Financial Involvement with a pharma- trolling for rs6971 genotype) between neuroinflammation ceutical or biotechnology company, a company providing (VTs) and cortical thickness in the right medial prefrontal clinical assessment, scientific, or medical products or compa- cortex [r = -.562, p = .029] and the left dorsolateral nies doing business with or proposing to do business with prefrontal cortex [r = -.629, p = .012](p values are not ACNP over past 2 years (Calendar Years 2014–Present); Part 2: corrected for multiple comparisons). No significant associa- Income Sources & Equity of $10,000 per year or greater tions were found with surface area. (Calendar Years 2014 - Present): List those financial relation- Conclusions: These results, while preliminary, suggest links ships which are listed in part one and have a value greater than between the microglial activation/neuroinflammation and $10,000 per year, OR financial holdings that are listed in part cortical thickness in the dorsolateral prefrontal cortex and one and have a value of $10,000 or greater as of the date of medial prefrontal cortex in AD patients. -
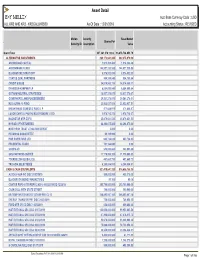
Asset Detail Acct Base Currency Code : USD ALL KR2 and KR3 - KR2GALLKRS00 As of Date : 12/31/2018 Accounting Status : REVISED
Asset Detail Acct Base Currency Code : USD ALL KR2 AND KR3 - KR2GALLKRS00 As Of Date : 12/31/2018 Accounting Status : REVISED Mellon Security Base Market . Shares/Par Security ID Description Value Grand Total 337,341,374,122.6.. 16,678,734,053.79 ALTERNATIVE INVESTMENTS 383,172,041.330 382,375,079.30 ANCHORAGE CAPITAL 1,974,352.480 1,974,352.48 ARROWMARK FUND I 144,927,230.580 144,927,230.58 BLACKSTONE STRAT OPP 8,576,532.030 8,576,532.03 COATUE QUAL PARTNERS 904,189.840 904,189.84 CREDIT SUISSE 34,074,860.110 34,074,860.11 DAVIDSON-KEMPNER LP 6,884,099.840 6,884,099.84 GOTHAM NEUTRAL STRATEGIES 18,837,376.670 18,837,376.67 GOVERNORS LANE FUNDÉÉÉÉÉÉÉÉ 29,561,278.010 29,561,278.01 H2O ALPHA-10 FUND 23,932,037.510 23,932,037.51 KNIGHTHEAD DOMESTIC FUND L P 471,648.970 471,648.97 LUXOR CAPITAL PARTNERS OFFSHORE LTDD 2,978,710.270 2,978,710.27 MAGNETAR MTP EOF II 25,879,631.530 25,879,631.53 MYRIAD OPPORTUNITIES 64,294,675.000 64,294,675.00 NORTHERN TRUST LITIGATION CREDIT 2.000 0.00 PERSHING SQUAREÉÉÉÉ 95,795.990 0.00 PINE RIVER FUND LTD 603,724.620 603,724.62 PRUDENTIAL FUND I 701,164.040 0.00 SCOPIA VII 548,095.880 548,095.88 SRS PARTNERS USÉÉÉÉ 11,179,090.320 11,179,090.32 TOURBILLON GLOBAL EQ 491,660.730 491,660.73 TRICADIA SELECTÉÉÉÉ 6,255,884.910 6,255,884.91 CASH & CASH EQUIVALENTS 927,419,647.320 916,686,158.35 AUTOLIV ASP INC DISC 01/02/2019 500,000.000 499,375.00 BLACKROCK MONEY MARKET FD B 90.160 90.16 CANTOR REPO A TRI REPO 2.450% 01/02/2019 DD 12/28/18 295,700,000.000 295,700,000.00 CASH COLL WITH STATE STREET 190,000.000 190,000.00 -

Merck & Co., Inc
As filed with the Securities and Exchange Commission on February 25, 2021 UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D. C. 20549 _________________________________ FORM 10-K (MARK ONE) ☒ Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the Fiscal Year Ended December 31, 2020 OR ☐ Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the transition period from to Commission File No. 1-6571 _________________________________ Merck & Co., Inc. 2000 Galloping Hill Road Kenilworth New Jersey 07033 (908) 740-4000 New Jersey 22-1918501 (State or other jurisdiction of incorporation) (I.R.S Employer Identification No.) Securities Registered pursuant to Section 12(b) of the Act: Title of Each Class Trading Symbol(s) Name of Each Exchange on which Registered Common Stock ($0.50 par value) MRK New York Stock Exchange 1.125% Notes due 2021 MRK/21 New York Stock Exchange 0.500% Notes due 2024 MRK 24 New York Stock Exchange 1.875% Notes due 2026 MRK/26 New York Stock Exchange 2.500% Notes due 2034 MRK/34 New York Stock Exchange 1.375% Notes due 2036 MRK 36A New York Stock Exchange Number of shares of Common Stock ($0.50 par value) outstanding as of January 31, 2021: 2,530,315,668. Aggregate market value of Common Stock ($0.50 par value) held by non-affiliates on June 30, 2020 based on closing price on June 30, 2020: $195,461,000,000. Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. -

Oregon Drug Use Review / Pharmacy & Therapeutics Committee
© Copyright 2012 Oregon State University. All Rights Reserved Drug Use Research & Management Program OHA Division of Medical Assistance Programs 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Oregon Drug Use Review / Pharmacy & Therapeutics Committee Thursday, July 26, 2018 1:00 - 5:00 PM HP Conference Room 4070 27th Ct. SE Salem, OR 97302 MEETING AGENDA NOTE: Any agenda items discussed by the DUR/P&T Committee may result in changes to utilization control recommendations to the OHA. Timing, sequence and inclusion of agenda items presented to the Committee may change at the discretion of the OHA, P&T Committee and staff. The DUR/P&T Committee functions as the Rules Advisory Committee to the Oregon Health Plan for adoption into Oregon Administrative Rules 410-121-0030 & 410-121-0040 as required by 414.325(9). I. CALL TO ORDER 1:00 PM A. Roll Call & Introductions R. Citron (OSU) B. Conflict of Interest Declaration R. Citron (OSU) C. Approval of Agenda and Minutes T. Klein (Chair) D. Department Update T. Douglass (OHA) E. Legislative Update T. Douglass (OHA) F. Mental Health Clinical Advisory Group Discussion K. Shirley (MHCAG) 1:40 PM II. CONSENT AGENDA TOPICS T. Klein (Chair) A. P&T Methods B. CMS and State Annual Reports C. Quarterly Utilization Reports 1. Public Comment III. DUR ACTIVITIES 1:45 PM A. ProDUR Report R. Holsapple (DXC) B. RetroDUR Report D. Engen (OSU) C. Oregon State Drug Reviews K. Sentena (OSU) 1. A Review of Implications of FDA Expedited Approval Pathways, Including the Breakthrough Therapy Designation IV. -

Brain Metabolic Profile After Intranasal Vs. Intraperitoneal Clomipramine
International Journal of Molecular Sciences Article Brain Metabolic Profile after Intranasal vs. Intraperitoneal Clomipramine Treatment in Rats with Ultrasound Model of Depression Olga Abramova 1,2, Yana Zorkina 1,2,* , Timur Syunyakov 2,3 , Eugene Zubkov 1, Valeria Ushakova 1,2, Artemiy Silantyev 1, Kristina Soloveva 2, Olga Gurina 1, Alexander Majouga 4, Anna Morozova 1,2 and Vladimir Chekhonin 1,5 1 V. Serbsky National Medical Research Centre of Psychiatry and Narcology, 119034 Moscow, Russia; [email protected] (O.A.); [email protected] (E.Z.); [email protected] (V.U.); [email protected] (A.S.); [email protected] (O.G.); [email protected] (A.M.); [email protected] (V.C.) 2 Mental-Health Clinic No. 1 Named after N.A. Alekseev, 117152 Moscow, Russia; [email protected] (T.S.); [email protected] (K.S.) 3 Federal State Budgetary Institution Research Zakusov Institute of Pharmacology, 125315 Moscow, Russia 4 Drug Delivery Systems Laboratory, D. Mendeleev University of Chemical Technology of Russia, Miusskaya pl. 9, 125047 Moscow, Russia; [email protected] 5 Department of Medical Nanobiotechnology, Pirogov Russian National Research Medical University, 117997 Moscow, Russia * Correspondence: [email protected]; Tel.: +7-916-588-4851 Citation: Abramova, O.; Zorkina, Y.; Abstract: Background: Molecular mechanisms of depression remain unclear. The brain metabolome Syunyakov, T.; Zubkov, E.; Ushakova, after antidepressant therapy is poorly understood and had not been performed for different routes V.; Silantyev, A.; Soloveva, K.; Gurina, of drug administration before the present study. Rats were exposed to chronic ultrasound stress O.; Majouga, A.; Morozova, A.; et al. and treated with intranasal and intraperitoneal clomipramine.