Periodic Table of the Elements 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Densifying Metal Hydrides with High Temperature and Pressure
3,784,682 United States Patent Office Patented Jan. 8, 1974 feet the true density. That is, by this method only theo- 3,784,682 retical or near theoretical densities can be obtained by DENSIFYING METAL HYDRIDES WITH HIGH making the material quite free from porosity (p. 354). TEMPERATURE AND PRESSURE The true density remains the same. Leonard M. NiebylsM, Birmingham, Mich., assignor to Ethyl Corporation, Richmond, Va. SUMMARY OF THE INVENTION No Drawing. Continuation-in-part of abandoned applica- tion Ser. No. 392,370, Aug. 24, 1964. This application The process of this invention provides a practical Apr. 9,1968, Ser. No. 721,135 method of increasing the true density of hydrides of Int. CI. COlb 6/00, 6/06 metals of Groups II-A, II-B, III-A and III-B of the U.S. CI. 423—645 8 Claims Periodic Table. More specifically, true densities of said 10 metal hydrides may be substantially increased by subject- ing a hydride to superatmospheric pressures at or above ABSTRACT OF THE DISCLOSURE fusion temperatures. When beryllium hydride is subjected A method of increasing the density of a hydride of a to this process, a material having a density of at least metal of Groups II-A, II-B, III-A and III-B of the 0.69 g./cc. is obtained. It may or may not be crystalline. Periodic Table which comprises subjecting a hydride to 15 a pressure of from about 50,000 p.s.i. to about 900,000 DESCRIPTION OF THE PREFERRED p.s.i. at or above the fusion temperature of the hydride; EMBODIMENT i.e., between about 65° C. -
Comparison of Sulfur to Oxygen*
OpenStax-CNX module: m34977 1 Comparison of Sulfur to Oxygen* Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 1 Size Table 1 summarizes the comparative sizes of oxygen and sulfur. Element Atomic radius Covalent radius Ionic radius (Å) van der Waal ra- (Å) (Å) dius (Å) Oxygen 0.48 0.66 1.40 1.52 Sulfur 0.88 1.05 1.84 1.80 Table 1: Comparison of physical characteristics for oxygen and sulfur. 2 Electronegativity Sulfur is less electronegative than oxygen (2.4 and 3.5, respectively) and as a consequence bonds to sulfur are less polar than the corresponding bonds to oxygen. One signicant result in that with a less polar S-H bond the subsequent hydrogen bonding is weaker than observed with O-H analogs. A further consequence of the lower electronegativity is that the S-O bond is polar. 3 Bonds formed Sulfur forms a range of bonding types. As with oxygen the -2 oxidation state prevalent. For example, sulfur forms analogs of ethers, i.e., thioethers R-S-R. However, unlike oxygen, sulfur can form more than two covalent (non-dative) bonds, i.e., in compounds such as SF4 and SF6. Such hypervalent compounds were originally thought be due to the inclusion of low energy d orbitals 3 2 in hybrids (e.g., sp d for SF6); however, a better picture involves a combination of s and p ortbitals in bonding (Figure 1). Any involvement of the d orbitals is limited to the polarization of the p orbitals rather than direct hydridization. -

Thermodynamic Hydricity of Small Borane Clusters and Polyhedral Closo-Boranes
molecules Article Thermodynamic Hydricity of Small Borane Clusters y and Polyhedral closo-Boranes Igor E. Golub 1,* , Oleg A. Filippov 1 , Vasilisa A. Kulikova 1,2, Natalia V. Belkova 1 , Lina M. Epstein 1 and Elena S. Shubina 1,* 1 A. N. Nesmeyanov Institute of Organoelement Compounds and Russian Academy of Sciences (INEOS RAS), 28 Vavilova St, 119991 Moscow, Russia; [email protected] (O.A.F.); [email protected] (V.A.K.); [email protected] (N.V.B.); [email protected] (L.M.E.) 2 Faculty of Chemistry, M.V. Lomonosov Moscow State University, 1/3 Leninskiye Gory, 119991 Moscow, Russia * Correspondence: [email protected] (I.E.G.); [email protected] (E.S.S.) Dedicated to Professor Bohumil Štibr (1940-2020), who unfortunately passed away before he could reach the y age of 80, in the recognition of his outstanding contributions to boron chemistry. Academic Editors: Igor B. Sivaev, Narayan S. Hosmane and Bohumír Gr˝uner Received: 6 June 2020; Accepted: 23 June 2020; Published: 25 June 2020 MeCN Abstract: Thermodynamic hydricity (HDA ) determined as Gibbs free energy (DG◦[H]−) of the H− detachment reaction in acetonitrile (MeCN) was assessed for 144 small borane clusters (up 2 to 5 boron atoms), polyhedral closo-boranes dianions [BnHn] −, and their lithium salts Li2[BnHn] (n = 5–17) by DFT method [M06/6-311++G(d,p)] taking into account non-specific solvent effect (SMD MeCN model). Thermodynamic hydricity values of diborane B2H6 (HDA = 82.1 kcal/mol) and its 2 MeCN dianion [B2H6] − (HDA = 40.9 kcal/mol for Li2[B2H6]) can be selected as border points for the range of borane clusters’ reactivity. -

Additional Teacher Background Chapter 4 Lesson 3, P. 295
Additional Teacher Background Chapter 4 Lesson 3, p. 295 What determines the shape of the standard periodic table? One common question about the periodic table is why it has its distinctive shape. There are actu- ally many different ways to represent the periodic table including circular, spiral, and 3-D. But in most cases, it is shown as a basically horizontal chart with the elements making up a certain num- ber of rows and columns. In this view, the table is not a symmetrical rectangular chart but seems to have steps or pieces missing. The key to understanding the shape of the periodic table is to recognize that the characteristics of the atoms themselves and their relationships to one another determine the shape and patterns of the table. ©2011 American Chemical Society Middle School Chemistry Unit 303 A helpful starting point for explaining the shape of the periodic table is to look closely at the structure of the atoms themselves. You can see some important characteristics of atoms by look- ing at the chart of energy level diagrams. Remember that an energy level is a region around an atom’s nucleus that can hold a certain number of electrons. The chart shows the number of energy levels for each element as concentric shaded rings. It also shows the number of protons (atomic number) for each element under the element’s name. The electrons, which equal the number of protons, are shown as dots within the energy levels. The relationship between atomic number, energy levels, and the way electrons fill these levels determines the shape of the standard periodic table. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -

Catalytic, Thermal, Regioselective Functionalization of Alkanes and Arenes with Borane Reagents
ACS Symposium Series 885, Activation and Functionalization of C-H Bonds, Karen I. Goldberg and Alan S. Goldman, eds. 2004. CHAP TER 8 Catalytic, Thermal, Regioselective Functionalization of Alkanes and Arenes with Borane Reagents John F. Hartwig Department of Chemistry, Yale University, New Haven, Connecticut 06520-8107 Work in the author’s group that has led to a regioselective catalytic borylation of alkanes at the terminal position is summarized. Early findings on the photochemical, stoichiometric functionalization of arenes and alkanes and the successful extension of this work to a catalytic functionalization of alkanes under photochemical conditions is presented first. The discovery of complexes that catalyze the functionalization of alkanes to terminal alkylboronate esters is then presented, along with mechanistic studies on these system and computational work on the stoichiometric reactions of isolated metal-boryl compounds with alkanes. Parallel results on the development of catalysts and a mechanistic understanding of the borylation of arenes under mild conditions to form arylboronate esters are also presented. © 2004 American Chemical Society 136 137 1. Introduction Although alkanes are considered among the least reactive organic molecules, alkanes do react with simple elemental reagents such as halogens and oxygen.1,2) Thus, the conversion of alkanes to functionalized molecules at low temperatures with control of selectivity and at low temperatures is a focus for development of catalytic processes.(3) In particular the conversion of an alkane to a product with a functional group at the terminal position has been a longstanding goal (eq. 1). Terminal alcohols such as n-butanol and terminal amines, such as hexamethylene diamine, are major commodity chemicals(4) that are produced from reactants several steps downstream from alkane feedstocks. -

The Modern Periodic Table Part II: Periodic Trends
K The Modern Periodic Table Part II: Periodic Trends November 13, 2014 ATOMIC RADIUS Period Trend *Across a period, Atomic Size DECREASES as Atomic Number INCREASES DECREASING SIZE Li Na K Rb Cs 2.2.0 1. 0 0 Group Trend *Within a group Atomic Size INCREASES as Atomic number INCREASES Trend In Classes of the Elements Across a Period Per Metals Metalloids Nonmetals 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 11 12 13 14 15 16 17 18 3 Na Mg Al Si P S Cl Ar Trend in Ionic Radius Definition of Ion: An ion is a charged particle. Ions form from atoms when they gain or lose electrons. Trend in Ionic Radius v An ion that is formed when an atom gains electrons is negatively charged (anion) v Nonmetal ions form this way v Examples: Cl- , F- v An ion that is formed when an atom loses electrons is positively charged (cation) v Metal ions form this way v Examples: Al+3 , Li+ v METALS: The ATOM is larger than the ion v NON METALS: The ION is larger than atom Trends in Ionic Radii v In general, as you move left to right across a period the size of the positive ion decreases v As you move down a group the size of both positive and negative ions increase Ionization Energy Trends Definition: the energy required to remove an electron from a gaseous atom v The energy required to remove the first electron from an atom is its first ionization energy v The energy required to remove the second electron is its second ionization energy v Second and third ionization energies are always larger than the first Period Trend *Across a period, IONIZATION ENERGY increases from left to right Group Trend *Within a group, the IONIZATION ENERGY Decreases down a column Octet Rule Definition: Eight electrons in the outermost energy level (valence electrons) is a particularly stable arrangement. -

Mechanism of Action of Sodium Hypochlorite ISSN 0103-6440113
Braz Dent J (2002) 13(2): 113-117 Mechanism of action of sodium hypochlorite ISSN 0103-6440113 Mechanism of Action of Sodium Hypochlorite Carlos ESTRELA1 Cyntia R.A. ESTRELA1 Eduardo Luis BARBIN2 Júlio César E. SPANÓ2 Melissa A. MARCHESAN2 Jesus D. PÉCORA2 1Faculty of Dentistry, Federal University of Goiás, Goiânia, GO, Brazil 2Faculty of Dentistry of Ribeirão Preto, University of São Paulo, Ribeirão Preto, SP, Brazil The choice of an irrigating solution for use in infected root canals requires previous knowledge of the microorganisms responsible for the infectious process as well as the properties of different irrigating solutions. Complex internal anatomy, host defenses and microorganism virulence are important factors in the treatment of teeth with asymptomatic apical periodontitis. Irrigating solutions must have expressive antimicrobial action and tissue dissolution capacity. Sodium hypochlorite is the most used irrigating solution in endodontics, because its mechanism of action causes biosynthetic alterations in cellular metabolism and phospholipid destruction, formation of chloramines that interfere in cellular metabolism, oxidative action with irreversible enzymatic inactivation in bacteria, and lipid and fatty acid degradation. The aim of this work is to discuss the mechanism of action of sodium hypochlorite based on its antimicrobial and physico-chemical properties. Key Words: sodium hypochlorite, irrigating solution, intracanal dressing. INTRODUCTION microbial agent to the infected site, adequate concen- tration of the agent, -

Silicate Weathering in Anoxic Marine Sediment As a Requirement for Authigenic Carbonate Burial T
Earth-Science Reviews 200 (2020) 102960 Contents lists available at ScienceDirect Earth-Science Reviews journal homepage: www.elsevier.com/locate/earscirev Silicate weathering in anoxic marine sediment as a requirement for authigenic carbonate burial T Marta E. Torresa,*, Wei-Li Hongb, Evan A. Solomonc, Kitty Millikend, Ji-Hoon Kime, James C. Samplef, Barbara M.A. Teichertg, Klaus Wallmannh a College of Earth, Ocean and Atmospheric Science, Oregon State University, Corvallis OR 97331, USA b Geological Survey of Norway, Trondheim, Norway c School of Oceanography, University of Washington, Seattle, WA 98195, USA d Bureau of Economic Geology, University of Texas at Austin, Austin, TX 78713, USA e Petroleum and Marine Research Division, Korea Institute of Geoscience and Mineral Resources, Daejeon, South Korea f School of Earth and Sustainability, Northern Arizona University, Flagstaff, AZ 86011, USA g Geologisch-Paläontologisches Institut, Wilhelms-Universität Münster, Corrensstr. 24, 48149 Münster, Germany h IFM-GEOMAR Leibniz Institute of Marine Sciences, Wischhofstrasse 1-3, 24148 Kiel, Germany ARTICLE INFO ABSTRACT Keywords: We emphasize the importance of marine silicate weathering (MSiW) reactions in anoxic sediment as funda- Silicate weathering mental in generating alkalinity and cations needed for carbonate precipitation and preservation along con- Authigenic carbonate tinental margins. We use a model that couples thermodynamics with aqueous geochemistry to show that the CO2 Organogenic dolomite released during methanogenesis results in a drop in pH to 6.0; unless these protons are buffered by MSiW, Alkalinity carbonate minerals will dissolve. We present data from two regions: the India passive margin and the active Carbon cycling subduction zone off Japan, where ash and/or rivers supply the reactive silicate phase, as reflected in strontium isotope data. -

Disinfection Session Objectives
Disinfection Session Objectives • To introduce the principal disinfectants that may be used and highlight key advantages and disadvantages of each • To emphasise the use of chlorination for routine disinfection. • To describe the process of chlorination and discuss the concepts of breakpoint chlorination, chlorine demand and outline basic chlorine chemistry. • To discuss the types of chlorine available and how these may be used for routine disinfection. WHO SEMINAR PACK FOR DRINKING-WATER QUALITY Disinfection Introduction All water supplies should be disinfected. This is aimed both at inactivating remaining bacteria before distribution and providing a residual disinfectant to inactivate bacteria introduced by any subsequent ingress of contaminated water during storage or distribution. At present, the principal disinfectant used worldwide is chlorine, although alternatives are being increasingly investigated and process such as ozonation are becoming more common. Chlorine is generally the disinfectant of choice as it is reasonably efficient, cheap and easy to handle. In all but the smallest water treatment plants, chlorine is added to water as either in aqueous solution (calcium hypochlorite or sodium hypochlorite) or chlorine gas. Smaller supplies may use tablets of hypochlorite. Other disinfectants include ozone, ultraviolet light and iodine. These all have disadvantages. UV is not a particularly effective disinfectant and it is difficult to expose water for sufficient time for disinfection to be effective. Neither ozone or UV provide a residual disinfectant and therefore offer no protection against recontamination in distribution. To overcome this, in some water supplies booster ozonation stations are set up along the distribution network. Both iodine and ozone are carcinogenic. There are also significant health and safety concerns, for operators, regarding the generation and application of ozone and chlorine (especially in the gaseous form). -

Periodic Trends in the Main Group Elements
Chemistry of The Main Group Elements 1. Hydrogen Hydrogen is the most abundant element in the universe, but it accounts for less than 1% (by mass) in the Earth’s crust. It is the third most abundant element in the living system. There are three naturally occurring isotopes of hydrogen: hydrogen (1H) - the most abundant isotope, deuterium (2H), and tritium 3 ( H) which is radioactive. Most of hydrogen occurs as H2O, hydrocarbon, and biological compounds. Hydrogen is a colorless gas with m.p. = -259oC (14 K) and b.p. = -253oC (20 K). Hydrogen is placed in Group 1A (1), together with alkali metals, because of its single electron in the valence shell and its common oxidation state of +1. However, it is physically and chemically different from any of the alkali metals. Hydrogen reacts with reactive metals (such as those of Group 1A and 2A) to for metal hydrides, where hydrogen is the anion with a “-1” charge. Because of this hydrogen may also be placed in Group 7A (17) together with the halogens. Like other nonmetals, hydrogen has a relatively high ionization energy (I.E. = 1311 kJ/mol), and its electronegativity is 2.1 (twice as high as those of alkali metals). Reactions of Hydrogen with Reactive Metals to form Salt like Hydrides Hydrogen reacts with reactive metals to form ionic (salt like) hydrides: 2Li(s) + H2(g) 2LiH(s); Ca(s) + H2(g) CaH2(s); The hydrides are very reactive and act as a strong base. It reacts violently with water to produce hydrogen gas: NaH(s) + H2O(l) NaOH(aq) + H2(g); It is also a strong reducing agent and is used to reduce TiCl4 to titanium metal: TiCl4(l) + 4LiH(s) Ti(s) + 4LiCl(s) + 2H2(g) Reactions of Hydrogen with Nonmetals Hydrogen reacts with nonmetals to form covalent compounds such as HF, HCl, HBr, HI, H2O, H2S, NH3, CH4, and other organic and biological compounds. -
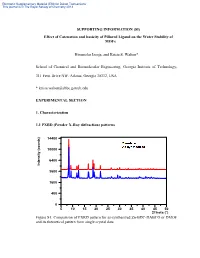
Effect of Catenation and Basicity of Pillared Ligand on the Water Stability of Mofs
Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 SUPPORTING INFORMATION (SI) Effect of Catenation and basicity of Pillared Ligand on the Water Stability of MOFs Himanshu Jasuja, and Krista S. Walton* School of Chemical and Biomolecular Engineering, Georgia Institute of Technology, 311 Ferst Drive NW, Atlanta, Georgia 30332, USA * [email protected] EXPERIMENTAL SECTION 1. Characterization 1.1 PXRD (Powder X-Ray diffraction) patterns 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S1: Comparison of PXRD pattern for as-synthesized Zn-BDC-DABCO or DMOF and its theoretical pattern from single crystal data Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S2: Comparison of PXRD pattern for as-synthesized Zn-BDC-BPY or MOF-508a and its theoretical pattern from single crystal data 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S3: Comparison of PXRD pattern for as-synthesized Zn-TMBDC-DABCO or DMOF-TM and its theoretical pattern from single crystal data Electronic Supplementary Material (ESI) for Dalton Transactions This journal is © The Royal Society of Chemistry 2013 14400 10000 6400 Intensity (counts) Intensity 3600 1600 400 0 5 10 15 20 25 30 35 40 45 50 2Theta (°) Figure S4: Comparison of PXRD pattern for as-synthesized Zn-TMBDC-BPY or MOF- 508-TM and its theoretical pattern from single crystal data 6400 3600 Intensity (counts) Intensity 1600 400 0 10 15 20 25 30 35 40 45 2Theta (°) Figure S5: PXRD patterns for as-synthesized Zn-BDC-BPY or MOF-508a and activated Zn-BDC-BPY or MOF-508b displaying shifting of peaks towards right on activation which was also observed by Chen et.