274 Barbary Dove
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lake Pinaroo Ramsar Site
Ecological character description: Lake Pinaroo Ramsar site Ecological character description: Lake Pinaroo Ramsar site Disclaimer The Department of Environment and Climate Change NSW (DECC) has compiled the Ecological character description: Lake Pinaroo Ramsar site in good faith, exercising all due care and attention. DECC does not accept responsibility for any inaccurate or incomplete information supplied by third parties. No representation is made about the accuracy, completeness or suitability of the information in this publication for any particular purpose. Readers should seek appropriate advice about the suitability of the information to their needs. © State of New South Wales and Department of Environment and Climate Change DECC is pleased to allow the reproduction of material from this publication on the condition that the source, publisher and authorship are appropriately acknowledged. Published by: Department of Environment and Climate Change NSW 59–61 Goulburn Street, Sydney PO Box A290, Sydney South 1232 Phone: 131555 (NSW only – publications and information requests) (02) 9995 5000 (switchboard) Fax: (02) 9995 5999 TTY: (02) 9211 4723 Email: [email protected] Website: www.environment.nsw.gov.au DECC 2008/275 ISBN 978 1 74122 839 7 June 2008 Printed on environmentally sustainable paper Cover photos Inset upper: Lake Pinaroo in flood, 1976 (DECC) Aerial: Lake Pinaroo in flood, March 1976 (DECC) Inset lower left: Blue-billed duck (R. Kingsford) Inset lower middle: Red-necked avocet (C. Herbert) Inset lower right: Red-capped plover (C. Herbert) Summary An ecological character description has been defined as ‘the combination of the ecosystem components, processes, benefits and services that characterise a wetland at a given point in time’. -

Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila Verreauxi)
diversity Article Diel and Seasonal Variations of Vocal Behavior of the Neotropical White-Tipped Dove (Leptotila verreauxi) Cristian Pérez-Granados 1,2,* and Karl-L. Schuchmann 1,3,4 1 National Institute for Science and Technology in Wetlands (INAU), Federal University of Mato Grosso (UFMT), Computational Bioacoustics Research Unit (CO.BRA), Fernando Correa da Costa Av. 2367, Cuiabá MT 78060-900, Brazil; [email protected] 2 Postgraduate Program in Ecology and Biodiversity Conservation, Institute of Biosciences, Federal University of Mato Grosso, Cuiabá MT 78060-900, Brazil 3 Zoological Research Museum A. Koenig (ZFMK), Ornithology, Adenauerallee 160, 53113 Bonn, Germany 4 Postgraduate Program in Zoology, Institute of Biosciences, Federal University of Mato Grosso, Cuiabá MT 78060-900, Brazil * Correspondence: [email protected] Received: 19 August 2020; Accepted: 14 October 2020; Published: 16 October 2020 Abstract: Current knowledge regarding the vocal behavior in tropical non-passerines is very limited. Here, we employed passive acoustic monitoring to study the vocal activity of the white-tipped dove (Leptotila verreauxi) at three sites over a year in the Brazilian Pantanal. The diel pattern of vocal activity showed a bimodal pattern, with significantly higher vocal activity after sunrise than during the other hours of the day, in agreement with prior studies on this species and other members of Columbidae. The species was vocally active throughout the year, but vocal activity was maximum during May-June and lowest during January-February. Relative air humidity was positively associated with vocal activity, which may be related to the improvement of sound transmission under more humid conditions, but it could also be related to foraging efficiency due to a higher availability of invertebrates on wetter days. -

Cravens Peak Scientific Study Report
Geography Monograph Series No. 13 Cravens Peak Scientific Study Report The Royal Geographical Society of Queensland Inc. Brisbane, 2009 The Royal Geographical Society of Queensland Inc. is a non-profit organization that promotes the study of Geography within educational, scientific, professional, commercial and broader general communities. Since its establishment in 1885, the Society has taken the lead in geo- graphical education, exploration and research in Queensland. Published by: The Royal Geographical Society of Queensland Inc. 237 Milton Road, Milton QLD 4064, Australia Phone: (07) 3368 2066; Fax: (07) 33671011 Email: [email protected] Website: www.rgsq.org.au ISBN 978 0 949286 16 8 ISSN 1037 7158 © 2009 Desktop Publishing: Kevin Long, Page People Pty Ltd (www.pagepeople.com.au) Printing: Snap Printing Milton (www.milton.snapprinting.com.au) Cover: Pemberton Design (www.pembertondesign.com.au) Cover photo: Cravens Peak. Photographer: Nick Rains 2007 State map and Topographic Map provided by: Richard MacNeill, Spatial Information Coordinator, Bush Heritage Australia (www.bushheritage.org.au) Other Titles in the Geography Monograph Series: No 1. Technology Education and Geography in Australia Higher Education No 2. Geography in Society: a Case for Geography in Australian Society No 3. Cape York Peninsula Scientific Study Report No 4. Musselbrook Reserve Scientific Study Report No 5. A Continent for a Nation; and, Dividing Societies No 6. Herald Cays Scientific Study Report No 7. Braving the Bull of Heaven; and, Societal Benefits from Seasonal Climate Forecasting No 8. Antarctica: a Conducted Tour from Ancient to Modern; and, Undara: the Longest Known Young Lava Flow No 9. White Mountains Scientific Study Report No 10. -
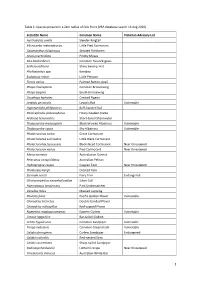
Scientific Name Common Name Victorian A
Table 1: Species present in a 2km radius of Crib Point (VBA database search 13 Aug 2020) Scientific Name Common Name Victorian Advisory List Austrolestes analis Slender Ringtail Microcarbo melanoleucos Little Pied Cormorant Calamanthus fuliginosus Striated Fieldwren Acacia verticillata Prickly Moses Poa labillardierei Common Tussock-grass Selliera radicans Shiny Swamp-mat Phyllostachys spp. Bamboo Eudyptula minor Little Penguin Turnix varius Painted Button-quail Phaps chalcoptera Common Bronzewing Phaps elegans Brush Bronzewing Ocyphaps lophotes Crested Pigeon Lewinia pectoralis Lewin's Rail Vulnerable Hypotaenidia philippensis Buff-banded Rail Poliocephalus poliocephalus Hoary-headed Grebe Ardenna tenuirostris Short-tailed Shearwater Thalassarche melanophris Black-browed Albatross Vulnerable Thalassarche cauta Shy Albatross Vulnerable Phalacrocorax carbo Great Cormorant Phalacrocorax sulcirostris Little Black Cormorant Phalacrocorax fuscescens Black-faced Cormorant Near threatened Phalacrocorax varius Pied Cormorant Near threatened Morus serrator Australasian Gannet Pelecanus conspicillatus Australian Pelican Hydroprogne caspia Caspian Tern Near threatened Thalasseus bergii Crested Tern Sternula nereis Fairy Tern Endangered Chroicocephalus novaehollandiae Silver Gull Haematopus longirostris Pied Oystercatcher Vanellus miles Masked Lapwing Pluvialis fulva Pacific Golden Plover Vulnerable Charadrius bicinctus Double-banded Plover Charadrius ruficapillus Red-capped Plover Numenius madagascariensis Eastern Curlew Vulnerable Limosa lapponica -

Abrolhos Painted Button-Quail (Turnix Varius Scintillans) Interim Recovery Plan
Abrolhos Painted Button-Quail (Turnix varius scintillans) Interim Recovery Plan Wildlife Management Program No. 63 Western Australia Department of Biodiversity, Conservation and Attractions May 2018 Wildlife Management Program No. 63 Abrolhos Painted Button-Quail (Turnix varius scintillans) Interim Recovery Plan Western Australia Department of Biodiversity, Conservation and Attractions Locked Bag 104, Bentley Delivery Centre, Western Australia 6983 Foreword Recovery plans are developed within the framework laid down in the Department of Biodiversity, Conservation and Attractions Corporate Policy Statement No. 35 (Parks and Wildlife, 2015b) and Corporate Guideline No. 36 (Parks and Wildlife, 2015a). Interim recovery plans outline the recovery actions that are needed to urgently address those threatening processes most affecting the ongoing survival of threatened taxa or ecological communities, and begin the recovery process. The attainment of objectives and the provision of funds necessary to implement actions are subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. This interim recovery plan was approved by the Department of Biodiversity, Conservation and Attractions, Western Australia. Approved interim recovery plans are subject to modification as dictated by new findings, changes in status of the taxon or ecological community, and the completion of recovery actions. Information in this interim recovery plan was accurate as of May 2018. Interim recovery plan preparation: -

Crested Barbary Dove (Streptopelia Risoria) in Pet Shop of Kushtia, Bangladesh
Journal of Dairy, Veterinary & Animal Research Short Communication Open Access Crested Barbary dove (streptopelia risoria) in pet shop of kushtia, Bangladesh Short communication Volume 8 Issue 4 - 2019 Crested fancy pigeons or pigeons are very common in Bangladesh but this is rare in doves. A shop in Kushtia district of Bangladesh, Ashraful Kabir M Department of Biology, Saidpur Cantonment Public College, they collected one wild type but crested Barbary Dove from Khulna Bangladesh and another white crested form from an unknown locality. Crowned pigeons are not available in Bangladesh. Only in Chittagong, Correspondence: M Ashraful Kabir, Department of Biology, Comilla, and Dhaka there some birds were found. From the personal Saidpur Cantonment Public College, Bangladesh, communication with the rearers, they said that productivity of those Email crested pigeons is very slow. In nature, Topknot and Pheasant Pigeons Received: March 07, 2019 | Published: August 30, 2019 have tuft and occipital crest. History says, selective breeding of fancy pigeons in Egypt they produced lots of crested pigeon varieties but this was not common in dove. Crested Choiseul Pigeon was extinct and now only Australian Crested Dove have upright crest. Selective breeding may produce huge crests in dove. Pigeons have various pattern of feather which created abnormal size or position of the feathers.1 Huge feathers of head cover the head and eyes and feather Goura victoria, Western- Goura cristata and Southern- Goura in legs and feet is muff. Most of the time abnormal feathers can cause scheepmakeri) are still surviving in the world (Plates 2‒4). difficulties in feeding, perching, flying, and breeding. -

Best of the Baltic - Bird List - July 2019 Note: *Species Are Listed in Order of First Seeing Them ** H = Heard Only
Best of the Baltic - Bird List - July 2019 Note: *Species are listed in order of first seeing them ** H = Heard Only July 6th 7th 8th 9th 10th 11th 12th 13th 14th 15th 16th 17th Mute Swan Cygnus olor X X X X X X X X Whopper Swan Cygnus cygnus X X X X Greylag Goose Anser anser X X X X X Barnacle Goose Branta leucopsis X X X Tufted Duck Aythya fuligula X X X X Common Eider Somateria mollissima X X X X X X X X Common Goldeneye Bucephala clangula X X X X X X Red-breasted Merganser Mergus serrator X X X X X Great Cormorant Phalacrocorax carbo X X X X X X X X X X Grey Heron Ardea cinerea X X X X X X X X X Western Marsh Harrier Circus aeruginosus X X X X White-tailed Eagle Haliaeetus albicilla X X X X Eurasian Coot Fulica atra X X X X X X X X Eurasian Oystercatcher Haematopus ostralegus X X X X X X X Black-headed Gull Chroicocephalus ridibundus X X X X X X X X X X X X European Herring Gull Larus argentatus X X X X X X X X X X X X Lesser Black-backed Gull Larus fuscus X X X X X X X X X X X X Great Black-backed Gull Larus marinus X X X X X X X X X X X X Common/Mew Gull Larus canus X X X X X X X X X X X X Common Tern Sterna hirundo X X X X X X X X X X X X Arctic Tern Sterna paradisaea X X X X X X X Feral Pigeon ( Rock) Columba livia X X X X X X X X X X X X Common Wood Pigeon Columba palumbus X X X X X X X X X X X Eurasian Collared Dove Streptopelia decaocto X X X Common Swift Apus apus X X X X X X X X X X X X Barn Swallow Hirundo rustica X X X X X X X X X X X Common House Martin Delichon urbicum X X X X X X X X White Wagtail Motacilla alba X X -

Eurasian Collared-Doves in Ohio: the Background Coverage
Birdin" the Pits these types ol pits m southwestern Ohio al~ne, and less tha~1 5% get any 61.rdmg Eurasian Collared-doves in Ohio: The Background coverage. tfwe are finding blue grosbeaks m most of the pits that do get birder coverage. then how many are there in the hundreds of~it~ that don 't? . by Bill Whan Two other rare Ohio species have shown an affiliation to gravel pits m 223 E. Tulane Rd. southwestern Ohio. Lark sparrows, an accidental species in Ohio away from Columbus, OH 43202 Oak Openings, have been reported breeding or presum~d breeding at th.ree . southwestern Ohio locations since 1980. On two occasions they were discovered m [email protected] gravel pits. In J 987 a pair nes t e~ in the Mt. Nebo g r ~v~ l pit near Shawnee Lookout Park in Hamilton County. A patr was seen there again m 1990 but was not The Eurasian collared-dove Streplopelia decaoc/o has made history confinned breeding. ln late May and early June of2004 a lark sparrow was present in lands far beyond its likely origins in India. Sri Lanka. and Myanmar. Its at the Roxanna-New Burlington gravel pit and seen by many birders who came present range in North America is already as extensive as that for the rock to see the brown pelican. The third site, as mentioned before. was along a newly pigeon Columbo /ivia, and it has occupied its adopted territory far more constructed section of Blue Rock Rd. in Hamilton County. a site that exhibited quickly. -

Expert Report of Professor Woinarski
NOTICE OF FILING This document was lodged electronically in the FEDERAL COURT OF AUSTRALIA (FCA) on 18/01/2019 3:23:32 PM AEDT and has been accepted for filing under the Court’s Rules. Details of filing follow and important additional information about these are set out below. Details of Filing Document Lodged: Expert Report File Number: VID1228/2017 File Title: FRIENDS OF LEADBEATER'S POSSUM INC v VICFORESTS Registry: VICTORIA REGISTRY - FEDERAL COURT OF AUSTRALIA Dated: 18/01/2019 3:23:39 PM AEDT Registrar Important Information As required by the Court’s Rules, this Notice has been inserted as the first page of the document which has been accepted for electronic filing. It is now taken to be part of that document for the purposes of the proceeding in the Court and contains important information for all parties to that proceeding. It must be included in the document served on each of those parties. The date and time of lodgment also shown above are the date and time that the document was received by the Court. Under the Court’s Rules the date of filing of the document is the day it was lodged (if that is a business day for the Registry which accepts it and the document was received by 4.30 pm local time at that Registry) or otherwise the next working day for that Registry. No. VID 1228 of 2017 Federal Court of Australia District Registry: Victoria Division: ACLHR FRIENDS OF LEADBEATER’S POSSUM INC Applicant VICFORESTS Respondent EXPERT REPORT OF PROFESSOR JOHN CASIMIR ZICHY WOINARSKI Contents: 1. -

Co-Extinct and Critically Co-Endangered Species of Parasitic Lice, and Conservation-Induced Extinction: Should Lice Be Reintroduced to Their Hosts?
Short Communication Co-extinct and critically co-endangered species of parasitic lice, and conservation-induced extinction: should lice be reintroduced to their hosts? L AJOS R ÓZSA and Z OLTÁN V AS Abstract The co-extinction of parasitic taxa and their host These problems highlight the need to develop reliable species is considered a common phenomenon in the current taxonomical knowledge about threatened and extinct global extinction crisis. However, information about the parasites. Although the co-extinction of host-specific conservation status of parasitic taxa is scarce. We present a dependent taxa (mutualists and parasites) and their hosts global list of co-extinct and critically co-endangered is known to be a feature of the ongoing wave of global parasitic lice (Phthiraptera), based on published data on extinctions (Stork & Lyal, 1993; Koh et al., 2004; Dunn et al., their host-specificity and their hosts’ conservation status 2009), the magnitude of this threat is difficult to assess. according to the IUCN Red List. We list six co-extinct Published lists of threatened animal parasites only cover and 40 (possibly 41) critically co-endangered species. ixodid ticks (Durden & Keirans, 1996; Mihalca et al., 2011), Additionally, we recognize 2–4 species that went extinct oestrid flies (Colwell et al., 2009), helminths of Brazilian as a result of conservation efforts to save their hosts. vertebrates (Muñiz-Pereira et al., 2009) and New Zealand Conservationists should consider preserving host-specific mites and lice (Buckley et al., 2012). Our aim here is to lice as part of their efforts to save species. provide a critical overview of the conservation status of parasitic lice. -

Songs of the Wild: Temporal and Geographical Distinctions in the Acoustic Properties of the Songs of the Yellow-Breasted Chat
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Theses and Dissertations in Animal Science Animal Science Department 12-3-2007 Songs of the Wild: Temporal and Geographical Distinctions in the Acoustic Properties of the Songs of the Yellow-Breasted Chat Jackie L. Canterbury University of Nebraska - Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/animalscidiss Part of the Animal Sciences Commons Canterbury, Jackie L., "Songs of the Wild: Temporal and Geographical Distinctions in the Acoustic Properties of the Songs of the Yellow-Breasted Chat" (2007). Theses and Dissertations in Animal Science. 4. https://digitalcommons.unl.edu/animalscidiss/4 This Article is brought to you for free and open access by the Animal Science Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Theses and Dissertations in Animal Science by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. SONGS OF THE WILD: TEMPORAL AND GEOGRAPHICAL DISTINCTIONS IN THE ACOUSTIC PROPERTIES OF THE SONGS OF THE YELLOW-BREASTED CHAT by Jacqueline Lee Canterbury A DISSERTATION Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Doctor of Philosophy Major: Animal Science Under the Supervision of Professors Dr. Mary M. Beck and Dr. Sheila E. Scheideler Lincoln, Nebraska November, 2007 SONGS OF THE WILD: TEMPORAL AND GEOGRAPHICAL DISTINCTIONS IN ACOUSTIC PROPERTIES OF SONG IN THE YELLOW-BREASTED CHAT Jacqueline Lee Canterbury, PhD. University of Nebraska, 2007 Advisors: Mary M. Beck and Sheila E. Scheideler The Yellow-breasted Chat, Icteria virens, is a member of the wood-warbler family, Parulidae, and exists as eastern I. -

Alectoris Chukar
PEST RISK ASSESSMENT Chukar partridge Alectoris chukar (Photo: courtesy of Olaf Oliviero Riemer. Image from Wikimedia Commons under a Creative Commons Attribution License, Version 3.) March 2011 This publication should be cited as: Latitude 42 (2011) Pest Risk Assessment: Chukar partridge (Alectoris chukar). Latitude 42 Environmental Consultants Pty Ltd. Hobart, Tasmania. About this Pest Risk Assessment This pest risk assessment is developed in accordance with the Policy and Procedures for the Import, Movement and Keeping of Vertebrate Wildlife in Tasmania (DPIPWE 2011). The policy and procedures set out conditions and restrictions for the importation of controlled animals pursuant to s32 of the Nature Conservation Act 2002. For more information about this Pest Risk Assessment, please contact: Wildlife Management Branch Department of Primary Industries, Parks, Water and Environment Address: GPO Box 44, Hobart, TAS. 7001, Australia. Phone: 1300 386 550 Email: [email protected] Visit: www.dpipwe.tas.gov.au Disclaimer The information provided in this Pest Risk Assessment is provided in good faith. The Crown, its officers, employees and agents do not accept liability however arising, including liability for negligence, for any loss resulting from the use of or reliance upon the information in this Pest Risk Assessment and/or reliance on its availability at any time. Pest Risk Assessment: Chukar partridge Alectoris chukar 2/20 1. Summary The chukar partridge (Alectoris chukar) is native to the mountainous regions of Asia, Western Europe and the Middle East (Robinson 2007, Wikipedia 2009). Its natural range includes Turkey, the Mediterranean islands, Iran and east through Russia and China and south into Pakistan and Nepal (Cowell 2008).