Conservation in the Wake of Myrtle Rust – a Case Study on Two Critically Endangered Australian Rainforest Plants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TML Propagation Protocols
PROPAGATION PROTOCOLS This document is intended as a guide for Tamborine Mountain Landcare members who wish to assist our regeneration projects by growing some of the plants needed. It is a work in progress so if you have anything to add to the protocols – for example a different but successful way of propagating and growing a particular plant – then please give it to Julie Lake so she can add it to the document. The idea is that our shared knowledge and experience can become a valuable part of TML's intellectual property as well as a useful source of knowledge for members. As there are many hundreds of plants native to Tamborine Mountain, the protocols list will take a long time to complete, with growing information for each plant added alphabetically as time permits. While the list is being compiled by those members with competence in this field, any TML member with a query about propagating a particular plant can post it on the website for other me mb e r s to answer. To date, only protocols for trees and shrubs have been compiled. Vines and ferns will be added later. Fruiting times given are usual for the species but many rainforest plants flower and fruit opportunistically, according to weather and other conditions unknown to us, thus fruit can be produced at any time of year. Finally, if anyone would like a copy of the protocols, contact Julie on [email protected] and she’ll send you one. ………………….. Growing from seed This is the best method for most plants destined for regeneration projects for it is usually fast, easy and ensures genetic diversity in the regenerated landscape. -

Five Hundred Plant Species in Gunung Halimun Salak National Park, West Java a Checklist Including Sundanese Names, Distribution and Use
Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman © 2010 Center for International Forestry Research. All rights reserved. Printed in Indonesia ISBN: 978-602-8693-22-6 Priyadi, H., Takao, G., Rahmawati, I., Supriyanto, B., Ikbal Nursal, W. and Rahman, I. 2010 Five hundred plant species in Gunung Halimun Salak National Park, West Java: a checklist including Sundanese names, distribution and use. CIFOR, Bogor, Indonesia. Photo credit: Hari Priyadi Layout: Rahadian Danil CIFOR Jl. CIFOR, Situ Gede Bogor Barat 16115 Indonesia T +62 (251) 8622-622 F +62 (251) 8622-100 E [email protected] www.cifor.cgiar.org Center for International Forestry Research (CIFOR) CIFOR advances human wellbeing, environmental conservation and equity by conducting research to inform policies and practices that affect forests in developing countries. CIFOR is one of 15 centres within the Consultative Group on International Agricultural Research (CGIAR). CIFOR’s headquarters are in Bogor, Indonesia. It also has offices in Asia, Africa and South America. | iii Contents Author biographies iv Background v How to use this guide vii Species checklist 1 Index of Sundanese names 159 Index of Latin names 166 References 179 iv | Author biographies Hari Priyadi is a research officer at CIFOR and a doctoral candidate funded by the Fonaso Erasmus Mundus programme of the European Union at Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences. -

The Biology of Casmara Subagronoma (Lepidoptera
insects Article The Biology of Casmara subagronoma (Lepidoptera: Oecophoridae), a Stem-Boring Moth of Rhodomyrtus tomentosa (Myrtaceae): Descriptions of the Previously Unknown Adult Female and Immature Stages, and Its Potential as a Biological Control Candidate Susan A. Wineriter-Wright 1, Melissa C. Smith 1,* , Mark A. Metz 2 , Jeffrey R. Makinson 3 , Bradley T. Brown 3, Matthew F. Purcell 3, Kane L. Barr 4 and Paul D. Pratt 5 1 USDA-ARS Invasive Plant Research Laboratory, Fort Lauderdale, FL 33314, USA; [email protected] 2 USDA-ARS Systematic Entomology Lab, Beltsville, MD 20013-7012, USA; [email protected] 3 USDA-ARS Australian Biological Control Laboratory, CSIRO Health and Biosecurity, Dutton Park QLD 4102, Australia; jeff[email protected] (J.R.M.); [email protected] (B.T.B.); [email protected] (M.F.P.) 4 USDA-ARS Center for Medical, Agricultural and Veterinary Entomology, Gainesville, FL 32608, USA; [email protected] 5 USDA-ARS, Western Regional Research Center, Invasive Species and Pollinator Health Research Unit, 800 Buchanan Street, Albany, CA 94710, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-954-475-6549 Received: 27 August 2020; Accepted: 16 September 2020; Published: 23 September 2020 Simple Summary: Rhodomyrtus tomentosa is a perennial woody shrub throughout Southeast Asia. Due to its prolific flower and fruit production, it was introduced into subtropical areas such as Florida and Hawai’i, where it is now naturalized and invasive. In an effort to find sustainable means to control R. tomentosa, a large-scale survey was mounted for biological control organisms. -

MEDIA FACTSHEET Habitat Enhancement Efforts at Pulau Ubin
MEDIA FACTSHEET Habitat enhancement efforts at Pulau Ubin Reforestation efforts at Tanjong Tajam, Pulau Ubin 100 native trees were planted at Tanjong Tajam, Pulau Ubin as part of habitat enhancement efforts on 23 August 2014. Minister of State for National Development, Desmond Lee, planted a Pteleocarpa lamponga. About the Pteleocarpa lamponga The Pteleocarpa lamponga is a medium- sized tree with a bushy, open crown that can grow to more than 30m tall. Its leaves have a leathery texture. Bright yellow flowers are found in clusters at the ends of leafy twigs. Native to Singapore, and also found in Thailand, Malaysia, Sumatra and Borneo, the Pteleocarpa lamponga is presumed to be extinct in the wild in Singapore. Page 1 of 9 Aphanamixis polystachya (Common names: Pasak Lingga, Amoora, Cikih, Kasai Paya, Kulim Burung) Growing up to 20m tall, the leaves of the Aphanamixis polystachya are oblong and leathery. Its sweetly scented flowers are cream, yellow or bronze in colour. Oil extracted from its seeds have medicinal properties and used externally for rheumatism. However, its fruits are poisonous. The Aphanamixis polystachya is classified as endangered in Singapore. Erythroxylum cuneatum (Common names: Wild Cocaine, Baka, Beluntas Bukit, Cinatamula, Inai Inai, Mahang Wangi, Medang Lenggundi, Medang Wangi) Page 2 of 9 Growing up to 45m tall, the Erythroxylum cuneatum Has flattened green twigs. Its tiny flowers grow in clusters of 1-8, and are white to light green in colour. Its fruits are bright red when ripe, and are eaten by mammals and porcupines. The Erythroxylum cuneatum is classified as common in Singapore and can be found in Changi, Pulau Tekong, Pulau Ubin and St John’s Island. -

In China: Phylogeny, Host Range, and Pathogenicity
Persoonia 45, 2020: 101–131 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/persoonia.2020.45.04 Cryphonectriaceae on Myrtales in China: phylogeny, host range, and pathogenicity W. Wang1,2, G.Q. Li1, Q.L. Liu1, S.F. Chen1,2 Key words Abstract Plantation-grown Eucalyptus (Myrtaceae) and other trees residing in the Myrtales have been widely planted in southern China. These fungal pathogens include species of Cryphonectriaceae that are well-known to cause stem Eucalyptus and branch canker disease on Myrtales trees. During recent disease surveys in southern China, sporocarps with fungal pathogen typical characteristics of Cryphonectriaceae were observed on the surfaces of cankers on the stems and branches host jump of Myrtales trees. In this study, a total of 164 Cryphonectriaceae isolates were identified based on comparisons of Myrtaceae DNA sequences of the partial conserved nuclear large subunit (LSU) ribosomal DNA, internal transcribed spacer new taxa (ITS) regions including the 5.8S gene of the ribosomal DNA operon, two regions of the β-tubulin (tub2/tub1) gene, plantation forestry and the translation elongation factor 1-alpha (tef1) gene region, as well as their morphological characteristics. The results showed that eight species reside in four genera of Cryphonectriaceae occurring on the genera Eucalyptus, Melastoma (Melastomataceae), Psidium (Myrtaceae), Syzygium (Myrtaceae), and Terminalia (Combretaceae) in Myrtales. These fungal species include Chrysoporthe deuterocubensis, Celoporthe syzygii, Cel. eucalypti, Cel. guang dongensis, Cel. cerciana, a new genus and two new species, as well as one new species of Aurifilum. These new taxa are hereby described as Parvosmorbus gen. -

Jervis Bay Territory Page 1 of 50 21-Jan-11 Species List for NRM Region (Blank), Jervis Bay Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -
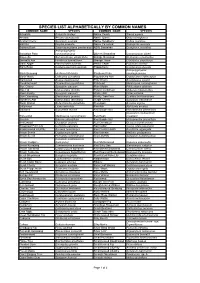
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Pre-Infection Stages of Austropuccinia Psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels
Article Pre-Infection Stages of Austropuccinia psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels Renata Ruiz Silva-Souza 1, André Costa da Silva 2, Roberto Antônio Rodella 3, José Eduardo Serrão 4, José Cola Zanuncio 5 and Edson Luiz Furtado 1,* 1 Departamento de Produção Vegetal, Faculdade de Ciências Agronômicas–UNESP, Botucatu 18610-307, São Paulo, Brasil; [email protected] 2 Departamento de Fitopatologia, Universidade Federal de Viçosa, Viçosa 36570-900, Minas Gerais, Brasil; [email protected] 3 Departamento de Botânica, Instituto de Biociências de Botucatu–UNESP, Botucatu 18618-000, São Paulo, Brasil; [email protected] 4 Departamento de Biologia Geral, Universidade Federal de Viçosa, Viçosa 36570-900, Minas Gerais, Brasil; [email protected] 5 Departamento de Entomologia/BIOAGRO, Universidade Federal de Viçosa, Viçosa 36570-900, Minas Gerais, Brasil; [email protected] * Correspondence: [email protected]; Tel.: +55-31-3899-2924 Received: 25 July 2017; Accepted: 20 September 2017; Published: 25 September 2017 Abstract: Rust is a major Eucalyptus spp. disease, which is especially damaging for early-stage plants. The aim of this study was to verify the pre-infection process of Austropuccinia psidii (A. psidii) in the leaves of three phenological stages of Eucalyptus clones with different resistance levels. Plants from the hybrids of Eucalyptus urophylla × Eucalyptus grandis (E. grandis) with variable levels of resistance to this disease were used. The pathogen was inoculated in vitro on abaxial leaf discs of first, third, and fifth leaf stages and maintained under conditions suitable for disease development. Subsequently, samples from these discs were collected 24 and 120 h after inoculation and processed using scanning electron microscopy analysis. -

Table of Contents Below) with Family Name Provided
1 Australian Plants Society Plant Table Profiles – Sutherland Group (updated August 2021) Below is a progressive list of all cultivated plants from members’ gardens and Joseph Banks Native Plants Reserve that have made an appearance on the Plant Table at Sutherland Group meetings. Links to websites are provided for the plants so that further research can be done. Plants are grouped in the categories of: Trees and large shrubs (woody plants generally taller than 4 m) Medium to small shrubs (woody plants from 0.1 to 4 m) Ground covers or ground-dwelling (Grasses, orchids, herbaceous and soft-wooded plants, ferns etc), as well as epiphytes (eg: Platycerium) Vines and scramblers Plants are in alphabetical order by botanic names within plants categories (see table of contents below) with family name provided. Common names are included where there is a known common name for the plant: Table of Contents Trees and Large shrubs........................................................................................................................... 2 Medium to small shrubs ...................................................................................................................... 23 Groundcovers and other ground‐dwelling plants as well as epiphytes. ............................................ 64 Vines and Scramblers ........................................................................................................................... 86 Sutherland Group http://sutherland.austplants.com.au 2 Trees and Large shrubs Acacia decurrens -

Austropuccinia Psidii
This diagnostic protocol was adopted by the Standards Committee on behalf of the Commission on Phytosanitary Measures in August 2018. The annex is a prescriptive part of ISPM 27. ISPM 27 Diagnostic protocols for regulated pests DP 26: Austropuccinia psidii Adopted 2018; published 2018 CONTENTS 1. Pest Information ...............................................................................................................................2 2. Taxonomic Information ....................................................................................................................2 3. Detection ...........................................................................................................................................4 3.1 Signs and symptoms of infection ......................................................................................4 3.2 Sampling and sample preparation .....................................................................................5 3.3 Morphological detection ...................................................................................................5 3.4 Molecular detection ...........................................................................................................6 3.4.1 Preparation of plant material .............................................................................................6 3.4.2 Nucleic acid extraction ......................................................................................................6 3.4.3 Conventional PCR and sequencing ...................................................................................7 -

Brisbane Native Plants by Suburb
INDEX - BRISBANE SUBURBS SPECIES LIST Acacia Ridge. ...........15 Chelmer ...................14 Hamilton. .................10 Mayne. .................25 Pullenvale............... 22 Toowong ....................46 Albion .......................25 Chermside West .11 Hawthorne................. 7 McDowall. ..............6 Torwood .....................47 Alderley ....................45 Clayfield ..................14 Heathwood.... 34. Meeandah.............. 2 Queensport ............32 Trinder Park ...............32 Algester.................... 15 Coopers Plains........32 Hemmant. .................32 Merthyr .................7 Annerley ...................32 Coorparoo ................3 Hendra. .................10 Middle Park .........19 Rainworth. ..............47 Underwood. ................41 Anstead ....................17 Corinda. ..................14 Herston ....................5 Milton ...................46 Ransome. ................32 Upper Brookfield .......23 Archerfield ...............32 Highgate Hill. ........43 Mitchelton ...........45 Red Hill.................... 43 Upper Mt gravatt. .......15 Ascot. .......................36 Darra .......................33 Hill End ..................45 Moggill. .................20 Richlands ................34 Ashgrove. ................26 Deagon ....................2 Holland Park........... 3 Moorooka. ............32 River Hills................ 19 Virginia ........................31 Aspley ......................31 Doboy ......................2 Morningside. .........3 Robertson ................42 Auchenflower -

Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae
molecules Review Structures and Bioactive Properties of Myrtucommulones and Related Acylphloroglucinols from Myrtaceae Rosario Nicoletti 1,2 , Maria Michela Salvatore 3 , Pasquale Ferranti 2 and Anna Andolfi 3,* 1 Council for Agricultural Research and Economics, Research Centre for Olive, Citrus and Tree Fruit, 81100 Caserta, Italy; [email protected] 2 Department of Agriculture, University of Naples ‘Federico II’, 80055 Portici, Italy; [email protected] 3 Department of Chemical Sciences, University of Naples ‘Federico II’, 80126 Naples, Italy; [email protected] * Correspondence: andolfi@unina.it; Tel.: +39-081-2539179 Academic Editors: Francesco Vinale and Maria Luisa Balestrieri Received: 2 December 2018; Accepted: 17 December 2018; Published: 19 December 2018 Abstract: Myrtaceae are a group of plants that include a number of renowned species used in ethnomedicine in many areas worldwide. Their valuable therapeutic properties have stimulated a fruitful research activity addressed to the identification of the bioactive components of their extracts yielding a great diversity of terpenes; polyphenols; and other exclusive products. Among the latter, starting with the discovery of myrtucommulone A from myrtle (Myrtus communis), a series of structurally-related acylphloroglucinol compounds have been characterized from several species that represent the basic active principles to be considered in view of possible drug development. Aspects concerning chemical and biological properties of these products are reviewed in the present paper. Keywords: myrtucommulone; acylphloroglucinols; Myrtaceae; plant extracts; biological activities 1. Introduction Myrtle (Myrtus communis) is a typical shrub of maquis and coastal bushes native of the Mediterranean area and Western Asia. It is well-known in traditional medicine, and for centuries its leaves and berries have found ethnomedical application in the treatment of several disorders of the digestive apparatus, as well as pulmonary and skin diseases [1,2].