Genetic Testing for Connective Tissue Disorders AHS – M2144
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Skin in the Ehlers-Danlos Syndromes
EDS Global Learning Conference July 30-August 1, 2019 (Nashville) The Skin in the Ehlers-Danlos Syndromes Dr Nigel Burrows Consultant Dermatologist MD FRCP Department of Dermatology Addenbrooke’s Hospital Cambridge University NHS Foundation Trust Cambridge, UK No conflict of interests or disclosures Burrows, N: The Skin in EDS 1 EDS Global Learning Conference July 30-August 1, 2019 (Nashville) • Overview of skin and anatomy • Skin features in commoner EDS • Skin features in rarer EDS subtypes • Skin management The skin • Is useful organ to sustain life ØProtection - microorganisms, ultraviolet light, mechanical damage ØSensation ØAllows movement ØEndocrine - vitamin D production ØExcretion - sweat ØTemperature regulation Burrows, N: The Skin in EDS 2 EDS Global Learning Conference July 30-August 1, 2019 (Nashville) The skin • Is useful organ to sustain life • Provides a visual clue to diagnoses • Important for cultures and traditions • Ready material for research Skin Fun Facts • Largest organ in the body • In an average adult the skin weighs approx 5kg (11lbs) and covers 2m2 (21 sq ft) • 11 miles of blood vessels • The average person has about 300 million skin cells • More than half of the dust in your home is actually dead skin • Your skin is home to more than 1,000 species of bacteria Burrows, N: The Skin in EDS 3 EDS Global Learning Conference July 30-August 1, 2019 (Nashville) The skin has 3 main layers Within the Dermis Extracellular Matrix 1. Collagen 2. Elastic fibres 3. Ground Substances i) glycosaminoglycans, ii) proteoglycans, -

Phenotype and Response to Growth Hormone Therapy in Siblings with B4GALT7 Deficiency T ⁎ Carla Sandler-Wilsona, Jennifer A
Bone 124 (2019) 14–21 Contents lists available at ScienceDirect Bone journal homepage: www.elsevier.com/locate/bone Case Report Phenotype and response to growth hormone therapy in siblings with B4GALT7 deficiency T ⁎ Carla Sandler-Wilsona, Jennifer A. Wambacha, , Bess A. Marshalla,b, Daniel J. Wegnera, William McAlisterc, F. Sessions Colea,b, Marwan Shinawia a Edward Mallinckrodt Department of Pediatrics, Washington University School of Medicine, St. Louis Children's Hospital, St. Louis, MO 63110, USA b Department of Cell Biology and Physiology, Washington University School of Medicine, St. Louis Children's Hospital, St. Louis, MO 63110, USA c Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis Children's Hospital, St. Louis, MO 63110, USA ARTICLE INFO ABSTRACT Keywords: B4GALT7 encodes beta-1,4-galactosyltransferase which links glycosaminoglycans to proteoglycans in connective B4GALT7 tissues. Rare, biallelic variants in B4GALT7 have been associated with spondylodysplastic Ehlers-Danlos and Spondylodysplastic Ehlers-Danlos syndrome Larsen of Reunion Island syndromes. Thirty patients with B4GALT7-related disorders have been reported to date Larsen of Reunion Island syndrome with phenotypic variability. Using whole exome sequencing, we identified male and female siblings with bial- Growth hormone lelic, pathogenic B4GALT7 variants and phenotypic features of spondylodysplastic Ehlers-Danlos syndrome as Proteoglycan well as previously unreported skeletal characteristics. We also provide detailed radiological -

Emaciation, Congestive Heart Failure, and Systemic Amyloidosis In
Case Report Emaciation, Congestive Heart Failure, and Systemic Amyloidosis in Severe Recessive Dystrophic Epidermolysis Bullosa: Possible Internal Complications Due to Skin-Derived Inflammatory Cytokines Derived from the Injured Skin Yoshiaki Matsushima y , Kento Mizutani y, Hiroyuki Goto y, Takehisa Nakanishi, Makoto Kondo, Koji Habe, Kenichi Isoda, Hitoshi Mizutani and Keiichi Yamanaka * Department of Dermatology, Mie University Graduate School of Medicine, Tsu, Mie 514-8507, Japan; [email protected] (Y.M.); [email protected] (K.M.); [email protected] (H.G.); [email protected] (T.N.); [email protected] (M.K.); [email protected] (K.H.); [email protected] (K.I.); [email protected] (H.M.) * Correspondence: [email protected]; Tel.: +81-59-231-5025; Fax: +81-59-231-5206 These authors contributed equally to this work. y Received: 2 August 2020; Accepted: 7 September 2020; Published: 14 September 2020 Abstract: Inherited epidermolysis bullosa (EB) is a rare genetic skin disorder characterized by epithelial tissue fragility. Recessive dystrophic epidermolysis bullosa (RDEB) is the most severe form, characterized by the presence of blisters, erosion, and ulcer formation, leading to scarring and contraction of the limbs. RDEB is also associated with extra-cutaneous complications, including emaciation, congestive heart failure, and systemic amyloidosis. The main cause of these clinical complications is unknown; however, we hypothesized that they are caused by elevated circulating inflammatory cytokines overproduced by injured keratinocytes. We addressed this phenomenon using keratin-14 driven, caspase-1 overexpressing, transgenic (KCASP1Tg) mice in which injured keratinocytes release high levels of IL-1α and β. -

Epidermolysis Bullosa (EB)
OR Preparation for Patients with Epidermolysis Bullosa (EB) Background: Patients with EB have a mutation in their keratin or collagen genes. As a result the skin is not properly anchored and mere touch can cause the skin to slough or blister. Many different subtypes have been identified but the most common variant we see in our patient population is recessive dystrophic EB. The children appear as burn patients and are frequently wrapped in total body burn dressings. NO ADHESIVES MAY BE USED IN THESE PATIENTS. You will note the following in these children. Airway: the tissues of the lining of the mouth, tongue and esophagus are affected making eating difficult. Mouth opening is SEVERELY LIMITED and these patients are always considered difficult airways. FOI is the preferred method to intubate since we try to minimize contact with the mucosa. Digits: continued skin slough and scarring results in absence of fingernails and the fingers often fuse together. Cardiac: Over time, some children develop a cardiomyopathy. Anemia is common as is continued infections, so these children often appear to be in a high-output state. Renal: Many children develop renal failure over time. Hemotologic: Continued bleeding from wounds and poor nutrition causes anemia. Infections: These children are often colonized with MRSA in their wounds and many act as though in low-grade sepsis. You will see tachycardia and anesthetic resistance. IV access: Difficult though not as impossible as you would imagine. NO ELASTIC TOURNIQUETS! A hand tourniquet is sufficient. Alcohol wipes are not usually used. A small amount of baby shampoo on moistened gauze dabbed on and off with moist gauze may be used GI tract: Esophageal strictures are common due to sloughing and scarring of the esophagus, so patients coming in for these procedures often cannot handle their oral secretions and are drooling. -

Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders
Title Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders Author(s) Mizumoto, Shuji; Kosho, Tomoki; Yamada, Shuhei; Sugahara, Kazuyuki Pharmaceuticals, 10(2), 34 Citation https://doi.org/10.3390/ph10020034 Issue Date 2017-06 Doc URL http://hdl.handle.net/2115/67053 Rights(URL) http://creativecommons.org/licenses/by/4.0/ Type article File Information pharmaceuticals10-2 34.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP pharmaceuticals Review Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders Shuji Mizumoto 1,*, Tomoki Kosho 2, Shuhei Yamada 1 and Kazuyuki Sugahara 1,* 1 Department of Pathobiochemistry, Faculty of Pharmacy, Meijo University, 150 Yagotoyama, Tempaku-ku, Nagoya 468-8503, Japan; [email protected] 2 Center for Medical Genetics, Shinshu University Hospital, 3-1-1 Asahi, Matsumoto, Nagano 390-8621, Japan; [email protected] * Correspondence: [email protected] (S.M.); [email protected] (K.S.); Tel.: +81-52-839-2652 Academic Editor: Barbara Mulloy Received: 22 February 2017; Accepted: 24 March 2017; Published: 27 March 2017 Abstract: The indispensable roles of dermatan sulfate-proteoglycans (DS-PGs) have been demonstrated in various biological events including construction of the extracellular matrix and cell signaling through interactions with collagen and transforming growth factor-β, respectively. Defects in the core proteins of DS-PGs such as decorin and -

Ichthyosis: Case Report in a Colombian Man with Genetic Alterations in ABCA12 and HRNR Genes Ruben D
Arias‑Pérez et al. BMC Med Genomics (2021) 14:140 https://doi.org/10.1186/s12920‑021‑00987‑y CASE REPORT Open Access Ichthyosis: case report in a Colombian man with genetic alterations in ABCA12 and HRNR genes Ruben D. Arias‑Pérez1, Salomón Gallego‑Quintero1, Natalia A. Taborda1 , Jorge E. Restrepo2, Renato Zambrano‑Cruz3 , William Tamayo‑Agudelo3, Patricia Bermúdez4, Constanza Duque4, Ismael Arroyave4, Johanna A. Tejada‑Moreno5, Andrés Villegas‑Lanau5, Alejandro Mejía‑García5, Wildeman Zapata6 , Juan C. Hernandez6* and Gina Cuartas‑Montoya3 Abstract Background: Ichthyosis is a heterogeneous group of diseases caused by genetic disorders related to skin formation. They are characterized by generalized dry skin, scaling, hyperkeratosis and frequently associated with erythroderma. Among its diferent types, harlequin ichthyosis (HI) stands out due to its severity. HI is caused by mutations in the ABCA12 gene, which encodes essential proteins in epidermal lipid transport, and it helps maintain the homeostasis of the stratum corneum of the epidermis. However, due to the wide spectrum of genetic alterations that can cause ichthyosis, holistic medical care, and genetic studies are required to improve the diagnosis and outcomes of these diseases. Case presentation: Here, we presented the case of a 19 years old male patient who was a premature infant and exhibited clinical features consistent with HI, including bright yellow hyperkeratotic plates with erythematous fssures that covered his entire body like a collodion baby. Currently, he exhibited erythroderma, photosensitivity, ectropion, auricular pavilion alterations, and musculoskeletal disorders, such as equinovarus feet, fngers, hands, and hypoplastic feet with contractures in fexion and marked difculty in fne motor skills. -

Blueprint Genetics Epidermolysis Bullosa Panel
Epidermolysis Bullosa Panel Test code: DE0301 Is a 26 gene panel that includes assessment of non-coding variants. Is ideal for patients with a clinical suspicion of congenital epidermolysis bullosa. About Epidermolysis Bullosa Epidermolysis bullosa (EB) is a group of inherited diseases that are characterised by blistering lesions on the skin and mucous membranes, most commonly appearing at sites of friction and minor trauma such as the feet and hands. In some subtypes, blisters may also occur on internal organs, such as the oesophagus, stomach and respiratory tract, without any apparent friction. There are 4 major types of EB based on different sites of blister formation within the skin structure: Epidermolysis bullosa simplex (EBS), Junctional epidermolysis bullosa (JEB), Dystrophic epidermolysis bullosa (DEB), and Kindler syndrome (KS). EBS is usually characterized by skin fragility and rarely mucosal epithelia that results in non-scarring blisters caused by mild or no trauma. The four most common subtypes of EBS are: 1) localized EBS (EBS-loc; also known as Weber-Cockayne type), 2) Dowling-Meara type EBS (EBS-DM), 3) other generalized EBS(EBS, gen-nonDM; also known as Koebner type) and 4) EBS-with mottled pigmentation (EBS-MP). Skin biopsy from fresh blister is considered mandatory for diagnostics of generalized forms of EBS. The prevalence of EBS is is estimated to be 1:30,000 - 50,000. EBS-loc is the most prevalent, EBS- DM and EBS-gen-nonDM are rare, and EBS-MP is even rarer. Penetrance is 100% for known KRT5 and KRT14 mutations. Location of the mutations within functional domains of KRT5and KRT14 has shown to predict EBS phenotype. -
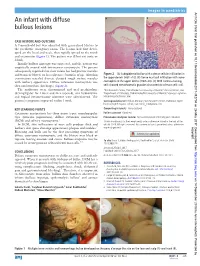
An Infant with Diffuse Bullous Lesions
Images in paediatrics Arch Dis Child: first published as 10.1136/archdischild-2017-312704 on 26 May 2017. Downloaded from An infant with diffuse bullous lesions CASE HISTORY, AND OUTCOME A 7-month-old boy was admitted with generalised blisters to the paediatric emergency room. The lesions had first devel- oped on the head and neck, then rapidly spread to the trunk and extremities (figure 1). The patient was ill but not toxic or febrile. Initially bullous impetigo was suspected, and the patient was empirically treated with intravenous vancomycin. The parents subsequently reported that their infant has had pruritic macules and transient blisters on his scalp since 5 months of age. Also skin Figure 2 (A) Subepidermal bullae with a dense cellular infiltration in examination revealed discrete elevated rough surface macules the upper dermis (H&E ×10). (B) Dense mast cell infiltration with some with leathery appearance. Diffuse cutaneous mastocytosis was eosinophils in the upper dermis (H&E×20). (C) With Giemsa staining, then confirmed on skin biopsy (figure 2). cells showed metachromatic granules characteristic of mast cells ×40. The antibiotics were discontinued and oral prednisolone 1Skin Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran (0.5 mg/kg/day for 1 week and then tapered), oral hydroxyzine 2Department of Pathology, Shahid Beheshti University of Medical Sciences, Loghman- and topical betamethasone ointment were administered. The Hakim Hospital, Tehran, Iran patient’s symptoms improved within 1 week. Correspondence to Dr Nikoo Mozafari, Skin Research Center, Shohada e Tajrish hospital, Tajrish Square, Tehran, Iran; nikoo_ md@ yahoo. com KEY LEARNING POINTS Competing interests None declared. -

How to Deal with Skin Biopsy in an Infant with Blisters?
Review How to Deal with Skin Biopsy in an Infant with Blisters? Stéphanie Leclerc-Mercier Reference Center for Genodermatoses (MAGEC Center), Department of Pathology, Necker-Enfants Malades Hospital, Paris Centre University, 75015 Paris, France; [email protected] Abstract: The onset of blisters in a neonate or an infant is often a source of great concern for both parents and physicians. A blistering rash can reveal a wide range of diseases with various backgrounds (infectious, genetic, autoimmune, drug-related, traumatic, etc.), so the challenge for the dermatologist and the pediatrician is to quickly determine the etiology, between benign causes and life-threatening disorders, for a better management of the patient. Clinical presentation can provide orientation for the diagnosis, but skin biopsy is often necessary in determining the cause of blister formations. In this article, we will provide information on the skin biopsy technique and discuss the clinical orientation in the case of a neonate or infant with a blistering eruption, with a focus on the histology for each etiology. Keywords: blistering eruption; infant; skin biopsy; genodermatosis; SSSS; hereditary epidermolysis bul- losa; keratinopathic ichthyosis; incontinentia pigmenti; mastocytosis; auto-immune blistering diseases 1. Introduction The onset of blisters in a neonate or an infant (<2 years old) is a source of great concern for both parents and physicians. Therefore, a precise diagnosis, between benign causes and Citation: Leclerc-Mercier, S. How to life-threatening disorders, is quickly needed for the best management of the baby. Deal with Skin Biopsy in an Infant Several diseases with various backgrounds (infectious, genetic, autoimmune, drug- with Blisters? Dermatopathology 2021, related, traumatic, etc.) can lead to a blistering eruption. -

Table I. Genodermatoses with Known Gene Defects 92 Pulkkinen
92 Pulkkinen, Ringpfeil, and Uitto JAM ACAD DERMATOL JULY 2002 Table I. Genodermatoses with known gene defects Reference Disease Mutated gene* Affected protein/function No.† Epidermal fragility disorders DEB COL7A1 Type VII collagen 6 Junctional EB LAMA3, LAMB3, ␣3, 3, and ␥2 chains of laminin 5, 6 LAMC2, COL17A1 type XVII collagen EB with pyloric atresia ITGA6, ITGB4 ␣64 Integrin 6 EB with muscular dystrophy PLEC1 Plectin 6 EB simplex KRT5, KRT14 Keratins 5 and 14 46 Ectodermal dysplasia with skin fragility PKP1 Plakophilin 1 47 Hailey-Hailey disease ATP2C1 ATP-dependent calcium transporter 13 Keratinization disorders Epidermolytic hyperkeratosis KRT1, KRT10 Keratins 1 and 10 46 Ichthyosis hystrix KRT1 Keratin 1 48 Epidermolytic PPK KRT9 Keratin 9 46 Nonepidermolytic PPK KRT1, KRT16 Keratins 1 and 16 46 Ichthyosis bullosa of Siemens KRT2e Keratin 2e 46 Pachyonychia congenita, types 1 and 2 KRT6a, KRT6b, KRT16, Keratins 6a, 6b, 16, and 17 46 KRT17 White sponge naevus KRT4, KRT13 Keratins 4 and 13 46 X-linked recessive ichthyosis STS Steroid sulfatase 49 Lamellar ichthyosis TGM1 Transglutaminase 1 50 Mutilating keratoderma with ichthyosis LOR Loricrin 10 Vohwinkel’s syndrome GJB2 Connexin 26 12 PPK with deafness GJB2 Connexin 26 12 Erythrokeratodermia variabilis GJB3, GJB4 Connexins 31 and 30.3 12 Darier disease ATP2A2 ATP-dependent calcium 14 transporter Striate PPK DSP, DSG1 Desmoplakin, desmoglein 1 51, 52 Conradi-Hu¨nermann-Happle syndrome EBP Delta 8-delta 7 sterol isomerase 53 (emopamil binding protein) Mal de Meleda ARS SLURP-1 -

The Ehlers-Danlos Syndromes, Rare Types
American Journal of Medical Genetics Part C (Seminars in Medical Genetics) 175C:70–115 (2017) RESEARCH REVIEW The Ehlers–Danlos Syndromes, Rare Types ANGELA F. BRADY, SERWET DEMIRDAS, SYLVIE FOURNEL-GIGLEUX, NEETI GHALI, CECILIA GIUNTA, INES KAPFERER-SEEBACHER, TOMOKI KOSHO, ROBERTO MENDOZA-LONDONO, MICHAEL F. POPE, MARIANNE ROHRBACH, TIM VAN DAMME, ANTHONY VANDERSTEEN, CAROLINE VAN MOURIK, NICOL VOERMANS, JOHANNES ZSCHOCKE, AND FRANSISKA MALFAIT * Dr. Angela F. Brady, F.R.C.P., Ph.D., is a Consultant Clinical Geneticist at the North West Thames Regional Genetics Service, London and she has a specialist interest in Ehlers–Danlos Syndrome. She was involved in setting up the UK National EDS Diagnostic Service which was established in 2009 and she has been working in the London part of the service since 2015. Dr. Serwet Demirdas, M.D., Ph.D., is a clinical geneticist in training at the Erasmus Medical Center (Erasmus University in Rotterdam, the Netherlands), where she is involved in the clinical service and research into the TNX deficient type of EDS. Prof. Sylvie Fournel-Gigleux, Pharm.D., Ph.D., is a basic researcher in biochemistry/pharmacology, Research Director at INSERM (Institut National de la Sante et de la Recherche Medicale) and co-head of the MolCelTEG Research Team at UMR 7561 CNRS-Universite de Lorraine. Her group is dedicated to the pathobiology of connective tissue disorders, in particular the Ehlers–Danlos syndromes, and specializes on the molecular and structural basis of glycosaminoglycan synthesis enzyme defects. Dr. Neeti Ghali, M.R.C.P.C.H., M.D., is a Consultant Clinical Geneticist at the North West Thames Regional Genetics Service, London and she has a specialist interest in Ehlers–Danlos Syndrome. -

Pathophysiological Significance of Dermatan
pharmaceuticals Review Pathophysiological Significance of Dermatan Sulfate Proteoglycans Revealed by Human Genetic Disorders Shuji Mizumoto 1,*, Tomoki Kosho 2, Shuhei Yamada 1 and Kazuyuki Sugahara 1,* 1 Department of Pathobiochemistry, Faculty of Pharmacy, Meijo University, 150 Yagotoyama, Tempaku-ku, Nagoya 468-8503, Japan; [email protected] 2 Center for Medical Genetics, Shinshu University Hospital, 3-1-1 Asahi, Matsumoto, Nagano 390-8621, Japan; [email protected] * Correspondence: [email protected] (S.M.); [email protected] (K.S.); Tel.: +81-52-839-2652 Academic Editor: Barbara Mulloy Received: 22 February 2017; Accepted: 24 March 2017; Published: 27 March 2017 Abstract: The indispensable roles of dermatan sulfate-proteoglycans (DS-PGs) have been demonstrated in various biological events including construction of the extracellular matrix and cell signaling through interactions with collagen and transforming growth factor-β, respectively. Defects in the core proteins of DS-PGs such as decorin and biglycan cause congenital stromal dystrophy of the cornea, spondyloepimetaphyseal dysplasia, and Meester-Loeys syndrome. Furthermore, mutations in human genes encoding the glycosyltransferases, epimerases, and sulfotransferases responsible for the biosynthesis of DS chains cause connective tissue disorders including Ehlers-Danlos syndrome and spondyloepimetaphyseal dysplasia with joint laxity characterized by skin hyperextensibility, joint hypermobility, and tissue fragility, and by severe skeletal disorders such as kyphoscoliosis, short trunk, dislocation, and joint laxity. Glycobiological approaches revealed that mutations in DS-biosynthetic enzymes cause reductions in enzymatic activities and in the amount of synthesized DS and also disrupt the formation of collagen bundles. This review focused on the growing number of glycobiological studies on recently reported genetic diseases caused by defects in the biosynthesis of DS and DS-PGs.