Assessment of Whitebark Pine Health on Eight National Forests in Oregon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Or Wilderness-Like Areas, but Instead Declassified Previously Protected Wildlands with High Timber Value
48 OREGON WILD A Brief Political History of Oregon’s Wilderness Protections Government protection should be thrown around every wild grove and forest on the Although the Forest Service pioneered the concept of wilderness protection in the mountains, as it is around every private orchard, and trees in public parks. To say 1920s and 1930s, by the late 1940s and 1950s, it was methodically undoing whatever nothing of their values as fountains of timber, they are worth infinitely more than all good it had done earlier by declassifying administrative wilderness areas that contained the gardens and parks of town. any commercial timber. —John Muir1 Just prior to the end of its second term, and after receiving over a million public comments in support of protecting national forest roadless areas, the Clinton Administration promulgated a regulation (a.k.a. “the Roadless Rule”) to protect the Inadequacies of Administrative remaining unprotected wildlands (greater than 5,000 acres in size) in the National Forest System from road building and logging. At the time, Clinton’s Forest Service Protections chief Mike Dombeck asked rhetorically: here is “government protection,” and then there is government protection. Mere public ownership — especially if managed by the Bureau of Is it worth one-quarter of 1 percent of our nation’s timber supply or a fraction of a Land Management — affords land little real or permanent protection. fraction of our oil and gas to protect 58.5 million acres of wild and unfragmented land T National forests enjoy somewhat more protection than BLM lands, but in perpetuity?2 to fully protect, conserve and restore federal forests often requires a combination of Wilderness designation and additional appropriate congressional Dombeck’s remarks echoed those of a Forest Service scientist from an earlier era. -

Vol. 23, No. the ORE.-BIN January 1961 STATE of OREGON Portland
THE ORE BIN Volume 23, 1961 Vol. 23, No. THE ORE.-BIN January 1961 STATE OF OREGON Portland, Oregon DEPARTMENT OF GEOLOGY AND MINERAL INDUSTRIES Head Office: 1069 State Office Bldg., Portland 1, Oregon Telephone: CApitol 6-2161, Ext. 488 State Governing Board Hollis M. Dole, Director William Kennedy, Chairman, Portland Harold Banta Baker Stoff Earl S. Mollard Riddle R. G. Bowen Geologist Field Offices R. E. Corcoran Geologist 2033 First Street, Boker L. L. Hoogland Assayer and Chemist N. S. Wagner, Field Geologist Rolph S. Mason Mining Engineer H. C. Brooks, Field Geologist T. C. Matthews Spectroscopist 239 S. E. "H" Street, Grants Pass V. C. Newton, Jr. Petroleum Engineer Len Ramp, Field Geologist H. G. Schlicker Geologist Norman Peterson, Field Geologist M. L. Steere Geologist * * * * * * * * * * * * * * * * * * OREGON MINERAL PRODUCTION IN 1960 By Ralph S. Mason* Oregon's mineral industry produced its second highest value of row minerals in 1960. Following a nation-wide economic trend during the year, the industry was off approximately $3 million from lost year's record-breaking high of $49.8 million, according to preliminary estimates mode by the U. S. Bureau of Mines. The heavy construction commodities, crushed stone and sand and grovel, reflected construction log and were responsible for most of the change from lost year. Metal mining, aside from nickel, was quiet. The state's only uronium mine and one of the two mercury producers shut down. Industrial mineral products showed both gains and losses as compared to the previous year. Cement production was up 12 percent while clays declined 15 percent and diatomite 3 percent. -

Record of Late Pleistocene Glaciation and Deglaciation in the Southern Cascade Range
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2004 Record of Late Pleistocene Glaciation and Deglaciation in the Southern Cascade Range. I. Petrological Evidence from Lacustrine Sediment in Upper Klamath Lake, Southern Oregon Richard L. Reynolds US Geological Survey Joseph G. Rosenbaum US Geological Survey Josh Rapp US Geological Survey Michael W. Kerwin US Geological Survey J. Platt Bradbury US Geological Survey See next page for additional authors Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Part of the Earth Sciences Commons Reynolds, Richard L.; Rosenbaum, Joseph G.; Rapp, Josh; Kerwin, Michael W.; Bradbury, J. Platt; Adam, David; and Colman, Steven, "Record of Late Pleistocene Glaciation and Deglaciation in the Southern Cascade Range. I. Petrological Evidence from Lacustrine Sediment in Upper Klamath Lake, Southern Oregon" (2004). USGS Staff -- Published Research. 255. https://digitalcommons.unl.edu/usgsstaffpub/255 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Authors Richard L. Reynolds, Joseph G. Rosenbaum, Josh Rapp, Michael W. Kerwin, J. Platt Bradbury, David Adam, and Steven Colman This article is available at DigitalCommons@University of Nebraska - Lincoln: https://digitalcommons.unl.edu/ usgsstaffpub/255 Journal of Paleolimnology 31: 217–233, 2004. 217 # 2004 Kluwer Academic Publishers. -
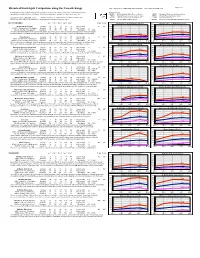
Historical Snowdepth Comparison Along the Cascade Range This Compilation Is ©2002-2005 Amar Andalkar
Page 1 of 5 Historical Snowdepth Comparison along the Cascade Range This compilation is ©2002-2005 Amar Andalkar www.skimountaineer.com Snowdepths in inches for the listed period of record, measured at snow courses, snow stakes, and automated gauges Data provided by: throughout the length of the Cascade Range. Note that snowfall data is available for only a few of these sites. BCRFC British Columbia River Forecast Centre NWAC Northwest Weather & Avalanche Center NRCS Natural Resources Conservation Service WRCC Western Regional Climate Center Updated through the 2003-2004 season. Italicized numbers are estimated based on limited available data. CCSS California Cooperative Snow Surveys NPS National Park Service See the end of the chart for an explanation of the Depth and Variability classification codes. WBSR Whistler Blackcomb Ski Resort ECNCA Environment Canada National Climate Archive BRITISH COLUMBIA Jan 1 Feb 1 Mar 1 Apr 1 May 1 Depth Variab 350 Bridge Glacier, N of Mt Meager (4600 ft) 350 Tenquille Lake, E of Mt Meager (5500 ft) Bridge Glacier (Lower) Average 47 61 65 69 61 Avg Snowfall — L LV 300 300 4600 ft (15 miles north of Mt Meager) Minimum 32 48 40 47 43 Max Snowfall — 250 250 BCRFC, monthly, 1995–present Maximum 63 88 117 108 87 Max Depth 117 (1999) 200 200 This fairly new measurement site is located at the eastern end of the Lillooet Icefield, in the next drainage north of the Mount Meager 171 150 150 Volcanic Complex. Snowdepths are relatively low but quite consistent due to its northerly location and icefield margin microclimate. -

10 Most Endangered Places 2011 an Oregon Wild Report
Oregon’s 10 Most Endangered Places 2011 an Oregon Wild Report 1 Oregon WIld 2010 10 Most Endangered Places Our mission: Since 1974, Oregon Wild has worked to protect and restore Oregon’s wildlands, wildlife, and waters as an enduring legacy for future generations. Editor: Sean Stevens Contributors: Submissions by: Lesley Adams Hells Canyon Preservation Council Erik Fernandez Klamath-Siskiyou Wildlands Center Chris Hansen Oregon Natural Desert Association Ani Kame’enui Rogue Riverkeeper Aaron Kilgore Umpqua Watersheds Rob Klavins Chandra LeGue Jennifer Schwartz Robin and Gerald Wisdom To find out more about our conservation work please visit www.oregonwild.org Copyright 2011, Oregon Wild *Fold out the cover for a spectacular Klamath Wetlands poster COVER: BRETT COLE 2 Oregon WIld 2010 ABOVE: ROGER DORBAND BRETT COLE TYLER ROEMER GREG TROUSLOT Congress attacks! Anti-environment extremists target Oregon’s wildlands When we last compiled the 10 Most Endangered Places The Wilderness and Roadless Release Act of 2011, report, national elections were poised to shake-up the proposed by Rep. Mike McCarthy (R-CA) and Sen. power dynamic in Washington, D.C. – and alter the John Barasso (R-WY), threatens no less than six of the future for much of America’s cherished public lands. special areas featured on these pages. Another rider aimed at exempting logging roads from the Clean Water The Tea Party movement that has steered the national Act puts at risk the Nestucca River (#2 on this year’s list) dialogue towards talk of deficits and debt ceilings has also and countless other waterways in Oregon. reframed the debate on the environment. -
Proposed Revised Designation of Critical Habitat for the Northern Spotted Owl (Strix Occidentalis Caurina); Proposed Rule
Tuesday, June 12, 2007 Part III Department of the Interior Fish and Wildlife Service 50 CFR Part 17 Endangered and Threatened Wildlife and Plants; Proposed Revised Designation of Critical Habitat for the Northern Spotted Owl (Strix occidentalis caurina); Proposed Rule VerDate Aug<31>2005 17:24 Jun 11, 2007 Jkt 211001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\12JNP3.SGM 12JNP3 rwilkins on PROD1PC63 with PROPOSALS3 32450 Federal Register / Vol. 72, No. 112 / Tuesday, June 12, 2007 / Proposed Rules DEPARTMENT OF THE INTERIOR Comments and materials received, as revised designation and, in particular, well as supporting documentation used any impacts on small entities; and the Fish and Wildlife Service in the preparation of this proposed rule, benefits of including or excluding areas will be available for public inspection, that exhibit these impacts; and 50 CFR Part 17 by appointment, during normal business (5) Whether any areas should or hours at the Oregon Fish and Wildlife should not be excluded from the revised RIN 1018–AU37 Office, at the address above; the Western designation under section 4(b)(2) of the Endangered and Threatened Wildlife Washington Fish and Wildlife Office, Act and why; and and Plants; Proposed Revised 510 Desmond Drive SE., Suite 101, (6) Whether our approach to designating critical habitat could be Designation of Critical Habitat for the Lacey, WA 98503; and the Yreka Fish improved or modified in any way to Northern Spotted Owl (Strix and Wildlife Office, 1829 S. Oregon St., provide for greater public participation occidentalis caurina) Yreka, CA 96097. and understanding, or to assist us in FOR FURTHER INFORMATION CONTACT: accommodating public concerns and AGENCY: Fish and Wildlife Service, Kemper McMaster, Field Supervisor, comments. -

Common Plants of the Upper Klamath Basin
Common Plants of the Upper Klamath Basin Technical Layout & Design .........Michael Calonje Editor ................................................Sarah Malaby Plant Descriptions & Text ..............Molly Juillerat, Ron Larson, Sarah Malaby, Jeanne Skalka. Photography ...........Michael Calonje, Ron Larson, Sarah Malaby, Terry Spivey. A Special acknowledgement to Klamath County Commissioners Al Switzer, John Elliott and Bill Brown for providing funding for publication costs through PL 06-393 Title III “Secure Rural Schools and Community Self-Determination Act of 2000” Oregon Native Plant Society - Klamath Basin Chapter Rabe Consulting 2007 CONTENTS Introduction ...................................................................................3 Overview ........................................................................................3 Habitats ..........................................................................................4 Plant Exploration in the Upper Klamath Basin .........................6 Growing Native Plants ..................................................................6 Species Groups ..............................................................................7 Ferns and Horsetails ..................................................................7 Conifers .....................................................................................8 Flowering Plants: Flowers, Hardwood Trees, and Shrubs .......9 Flowering Plants: Grasses and Grass-like Plants ...................9 Lichens, Bryophytes, and Blue-green -

Day 6 Ashland to Klamath Falls.Ai
Olson Mtn Seven Lakes 6884 5053 Medco Venus Pond Basin Weed Rd 7315 Agency Hill KLAMATH T JACKSON h r 4502 ee mile C 5038 Devils Peak r Camel Jupiter 7582 ROGUE RIVER 33-1E-25 7422 Hump eek e Lucifer r l 37 C o 6210 34 3105 ep Rustler 7481 Puck 3449 S a g i NATIONAL J a d o Lakes C Peak n o B Meva o c L u i Klamath r rk k 6208 g B o t a b t en Cr o l a f d h fo C l i R e s W l Lather Mtn w s l Braymill i o FOREST Agency a Cave r g a e C Luther Mtn r C 6945 r h 3484 s Mtn WNF t – C o 7153 el 4179 s s F h 5251 3450 K C 3260 i i e B M 949 Rd o ag d l i r ure o l g Santiam tt H l WINEMA d e M r L e q B a w l k a k e u Pine Ridge Tr u 200 c Peak a y p D O p i e N t r L n a Sky ke 4597 t 34-2E-29 34-3E-21 r Steiger el 4208 e C e Ch e err i y 3450 l Butte h W F i T 858 zzar 4785 d Lake r t RRNF Ish Cherry Peak e Fredenberg c erw e R oo d Butte p d L 6623 e s o ak 62 5815 n 4320 o g e r k La M b P r reek 32 u ke N C e o C ot Crater Lake Hwy – e a o Chiloquin Cre r s e r s F 35-3E-3 h Heavenly Twin el 4179 g a 3419 e B l d e C u l R k k o t a Lakes t r i e F y c WINEMA a v e Upper F i 3770 Lakes C s a e h r e Rock r l t 34 t P l t k s t r e a eek r o H u o h r l i C Butte c C a w B 32 n N Imagination y n 97 Smith NATIONAL t a n Round Top L 5810 #25#25 - 20122012 Falls i R ia H r Peak u Rock 3240 3690 e R 6495 E q h 6640 Klamath o c Red Lake el 4139 d l I Rancheria Rd y i Sout n h i ROGUE RIVER h L WNF ButteBut w c C te F T RRNF o S r n k O 3220 B FallsFalls e IVER 5805 W ilderness 980 R E C g Q E r el 22536536 W ODSODS RestRest K StopStop y e -

Forest Tree Seed Zones for Western Oregon (1996)
I I ( I ( ( ( ( . I ' ( I I ( I I • I FOREST TREE SEED ZONES FOR WESTERN OREGON (1996) ACKNOWLEDGMENTS I thank all reviewers for their valuable criticism and comments, most especially Drs. Frank C. Sorensen, Roy R. Silen, and W.Tom Adams. Mike Bordelon (Oregon Department of Forestry) I sincerely thank you for coordinating the overall project. Funds for this project were provided by U.S.D.A. Forest Service Cooperative Forestry and the Oregon Department of Forestry. Infonnation Compiled by William K. Randall U.S. Forest Service Printed by State of Oregon Department of Forestry 1996 Tree Seed Zones for Western Oregon INTRODUCTION 6 HISTORY OF SEED ZONES 8 DEVELOPING SEED ZONES 10 GENERAL SEED TRANSFER PRINCIPLES Introduction 13 SEED ORCHARD SEED 16 GUIDELINES FOR INDIVIDUAL SPECIES 18 Douglas-fir 18 Alaska Yellow Cedar 26 Incense Cedar 27 Port Orford Cedar 29 Western Red Cedar 30 Western Hemlock 32 Lodgepole Pine and Shore Pine 36 Ponderosa Pine 39 Jeffrey Pine 44 Sugar Pine 45 Western White Pine 48 Engelmann Spruce and Pacific Silver Fir 50 Sitka Spruce 52 Grand Fir, White Fir, and Interspecific Hybrids 54 Noble Fir / Shasta Fir 58 Pacific Yew 60 Red Alder 63 Black Cottonwood and Hybrid Poplars 67 Other Western Oregon Species 71 BIBLIOGRAPHY 75 ORDER FORM SEED ZONE MAPS Tree Seed Zones for Western Oregon INTRODUCTION The purpose of this document is to provide information to forest land managers and others about the level of risk associated with moving forest tree seed from a collection (source) environment to another location where it will be grown. -

Or Wild and Scenic Rivers System
Cascades Ecoregion ◆ Introduction 115 Young Volcanoes and Old Forests Cascades Ecoregion he Oregon portion of the Cascades Ecoregion encompasses 7.2 million growth forests. These include Roosevelt elk, black-tailed deer, beaver, black bear, coyote, acres and contains the highest mountains in the state. The Cascades marten, fisher, cougar, raccoon, rabbits, squirrels and (probably) lynx. Bird species Ecoregion is the backbone of Oregon, stretching lengthwise from the include the northern spotted owl and other owls, blue and ruffed grouse, band-tailed T Columbia River Gorge almost to the California border. Its width is pigeon, mountain quail, hawks, numerous songbirds, pileated woodpecker and other defined by the Willamette Valley and Klamath Mountains Ecoregions woodpeckers, bald eagle, golden eagle, osprey and peregrine falcon. Fish species include on the west and the Eastern Cascade Slope and Foothills Ecoregion on the east. The Pacific salmon stocks, bull trout and rainbow trout. Five of the eleven species endemic to highest peak is Mount Hood (11,239’). This ecoregion also extends northward into the ecoregion are amphibians: Pacific giant salamander, Cascade seep salamander, Washington and has three unusual outlier terrestrial “islands:” Paulina Mountains Oregon slender salamander, Larch Mountain salamander and the Cascades frog. southeast of Bend, Black Butte near Sisters and Mount Shasta in California. The effects of latitude on forest type are obvious in the Cascades as they range from Geologically, the ecoregion consists of two mountain ranges: the High Cascades the Columbia River to the California border. The effects of elevation are dramatic as well. and the Western (sometimes called “Old”) Cascades. Both are parallel north-south Beginning at the Willamette Valley margin and heading both eastward and ranges, but they are geologically distinct, as one is much older than the other. -

Notice Concerning Copyright Restrictions
NOTICE CONCERNING COPYRIGHT RESTRICTIONS This document may contain copyrighted materials. These materials have been made available for use in research, teaching, and private study, but may not be used for any commercial purpose. Users may not otherwise copy, reproduce, retransmit, distribute, publish, commercially exploit or otherwise transfer any material. The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specific conditions is that the photocopy or reproduction is not to be "used for any purpose other than private study, scholarship, or research." If a user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of "fair use," that user may be liable for copyright infringement. This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. - 4-1 r, Quaternary extrusion rates from the Cascade Range, northwestern United States and British Columbia David R. Sherrod and James G. Smith U.S. Geological Survey, Menlo Park, California 94025 ABSTRACT abruptly along the length of the volcanic arc. The The Quatemary extrusion rate and style of eruption Quaternary rate north of Mount Rainier is about 0.21 for the Cascade Range of thePacific Northwest changes km3/km/m.y.; in southern Washington and northern 13d 125• 120• - \ 1'.X 1 ..1-1• '' Mt. Garibaldi - 50' Explorer .0-9.RM&£14'Wl,6 . -

Chapter 3 Response to Issues, Concerns, and Opportunities Chapter 3 Response to Issues, Concerns, and Opportunities Introduction
Chapter 3 Response to Issues, Concerns, and Opportunities Chapter 3 Response to Issues, Concerns, and Opportunities Introduction The Forest consists of complex environmental conditions. Different individuals and groups would prefer to see the Forest managed to emphasize varying outputs, uses, and conditions. Management to emphasize some resources can cause changes in others. These are called trade-offs, and trade-offs accommodate differing demands, because there are practical and natural limlts to what the Forest can provide Different preferences of individuals and groups are represented in the issues and concerns that guide the forest planning process. A public issue is a subject of widespread public interest relating to management of the National Forest System. A management concern is an issue, a problem, or a condition identified by the Forest Service that affects the range of management activities in the planning process. A third component that influences alternatives comes from the various resource use and development opportunlties suggested by both the public and the Forest Service These opportunities are the basis of many of the issues and concerns used in this planning process. The opportunity to preselve or develop the resources of the Forest is the focus of the alternatives. Alternatives developed to respond to public issues are displayed in chapter 2 of the FEE Additional information concerning the development of this list of issues may be found in appendix A of the FEIS. Public Issues This Forest Plan was developed in response to the following public issues’ Pelican Butte Management of Former Klamath Indian Reservation Lands Timber Management Mule Deer Population Levels Recreation Management Wildlife Habitat Management Firewood Availability and Accessibility Mountain Pine Beetle Infestation Each public issue is phrased as a question to be addressed in the planning process.