Can Samba and Forró Brazilian Rhythmic Dance Be More Effective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Danças Urbanas Origem
GOVERNO DO ESTADO DO PARÁ SECRETARIA DO ESTADO DE EDUCAÇÃO A.O.S. DIOCESE DE ABAETETUBA EEEFM SÃO FRANCISCO XAVIER COMPONENTE CURRICULAR: EDUCAÇÃO FÍSICA ANO/SÉRIE: : 8° ANO PROFESSORES(AS)FISICAAA_____________ RESPONSAVEIS: Rui Pimentel Data Assunto Páginas Atividade 12/04 Aula 1: Danças urbanas origem. 01, 02 e Fazer a leitura e resolver a a História das danças urbanas do 03 atividade 1 16/04 Brasil. 19/04 Aula 2: Principais estilos de 03, 04 e Fazer a leitura e resolver a a dança 05 atividade 2 23/04 26/04 Danças de salão 05, 06, Fazer a leitura e resolver a a 07, 08 atividade 3 30/04 Danças de salão continuação 09, 10, Fazer a leitura e resolver a 11, 12 atividade 4 Danças Urbanas As danças urbanas estão presentes nos guetos e centros urbanos das cidades. Também aparecem na televisão, nos clipes, novelas, filmes, desenhos e academias. Assim como as coreografias do Tik Tok, performances urbanas estão mais popularizadas e ganhando novos adeptos. 1 Portanto, são manifestações culturais da força e identidade de grupos sociais. Mas também reúnem força e aceitação. Assim, elas têm grande papel na sociedade, de caráter pessoal e coletivo. E essa grande expressão é registrada através dos movimentos. A dança urbana foi a primeira manifestação corporal do emocional humano. Assim como outras danças, as danças urbanas também seguem regras próprias. Mas a liberdade em execução dos passos é a essência deste estilo. Origem As origens das danças urbanas são diversas. Uma delas ocorreu nos Estados Unidos, no século 20. Era uma manifestação dos artistas de rua, que estavam desempregados. -

Types of Dance Styles
Types of Dance Styles International Standard Ballroom Dances Ballroom Dance: Ballroom dancing is one of the most entertaining and elite styles of dancing. In the earlier days, ballroom dancewas only for the privileged class of people, the socialites if you must. This style of dancing with a partner, originated in Germany, but is now a popular act followed in varied dance styles. Today, the popularity of ballroom dance is evident, given the innumerable shows and competitions worldwide that revere dance, in all its form. This dance includes many other styles sub-categorized under this. There are many dance techniques that have been developed especially in America. The International Standard recognizes around 10 styles that belong to the category of ballroom dancing, whereas the American style has few forms that are different from those included under the International Standard. Tango: It definitely does take two to tango and this dance also belongs to the American Style category. Like all ballroom dancers, the male has to lead the female partner. The choreography of this dance is what sets it apart from other styles, varying between the International Standard, and that which is American. Waltz: The waltz is danced to melodic, slow music and is an equally beautiful dance form. The waltz is a graceful form of dance, that requires fluidity and delicate movement. When danced by the International Standard norms, this dance is performed more closely towards each other as compared to the American Style. Foxtrot: Foxtrot, as a dance style, gives a dancer flexibility to combine slow and fast dance steps together. -

The Dance Series from Ortofon Is Now Complete – MC Tango Joins MC Samba & MC Salsa for Almost Sixty Years, Ortofon Has
March, 2007 The dance series from Ortofon is now complete – MC Tango joins MC Samba & MC Salsa For almost sixty years, Ortofon has been at the forefront of music reproduction from analogue records. Throughout the years Ortofon have developed many new and creative designs for the enjoyment of music lovers and audio enthusiasts all over the world. WHY AN ORTOFON MOVING COIL CARTIDGE? Many have asked the question: Why is the sound of a moving coil cartridge so superior to that of a traditional magnetic: The answer in one word is linearity. The patented Ortofon low-impedance, low-output design is inherently linear over the entire audible range. This coupled with unequalled tracking ability and unique diamond profiles combines to deliver a sound previously deemed unattainable. The MC Tango, MC Samba and MC Salsa is a direct result of our endeavours. MC Tango has an elliptical diamond which is cut and polished to Ortofon’s standards, and the output is 0.5 mV. The MC Tango is our MC entry level cartridge with a very high cost/performance ratio. MC Samba and MC Salsa have a tiny, nude Elliptical or Fine Line diamond, mounted on a very light and rigid cantilever. This results in low equivalent stylus tip mass and cantilever compliance on 14 and 15 µm/mN, which means that MC Samba and MC Salsa will give excellent results with virtually any tone arm. The armature has been designed as a tiny lightweight cross, and the coils are mounted at exactly 90 degree to each other (just like the walls of the record groove), which is improving the channel separation. -

Brazilian Choro
The Brazilian by Tadeu Coelho and Julie Koidin Choro: Historical Perspectives and Performance Practices alanço is to choro as swing is to jazz—in both, mandatory elements to proper performance Band enjoyment of the music. Immersion in the sound of choro is imperative to playing it well. Knowledge of its origins and history is also helpful. Introduction the melody through spirited improvisations, sometimes David Willoughby, editor of the College Music Society quoting other melodies, from popular to classical styles. Newsletter, posed these questions: Should it not be a con- Although easier to decipher these performance intricacies stantly sought after goal for musicians trained in narrow via recordings, it still remains difficult—although not specialties to work together towards broader musical impossible—to catch the “twinkle” in the performer’s eye. understandings and towards the creation of a more Choro’s limited dissemination is furthered by its lack of vibrant musical culture? Should such a culture comprise accurate printed music. The vast majority of sheet music only materials imported from Western Europe? Should it publications have accompaniment that is written in a lead not synthesize musical repertories, of various kinds, from sheet format, i.e. chord symbols over melody. Without a all over the world?1 recording, it would be impossible to decipher the rhythms Throughout the world, the tradition of a country studying used in the accompaniment. The numerous errors found in its own cultural practices is not inceptive with its art. Such is the majority of publications, both in the melodic lines and the case of the choro, an indigenous music of Brazil, mostly chord symbols, further infringe on the probability of the instrumental, but at times with lyrics. -

Samba, Rumba, Cha-Cha, Salsa, Merengue, Cumbia, Flamenco, Tango, Bolero
SAMBA, RUMBA, CHA-CHA, SALSA, MERENGUE, CUMBIA, FLAMENCO, TANGO, BOLERO PROMOTIONAL MATERIAL DAVID GIARDINA Guitarist / Manager 860.568.1172 [email protected] www.gozaband.com ABOUT GOZA We are pleased to present to you GOZA - an engaging Latin/Latin Jazz musical ensemble comprised of Connecticut’s most seasoned and versatile musicians. GOZA (Spanish for Joy) performs exciting music and dance rhythms from Latin America, Brazil and Spain with guitar, violin, horns, Latin percussion and beautiful, romantic vocals. Goza rhythms include: samba, rumba cha-cha, salsa, cumbia, flamenco, tango, and bolero and num- bers by Jobim, Tito Puente, Gipsy Kings, Buena Vista, Rollins and Dizzy. We also have many originals and arrangements of Beatles, Santana, Stevie Wonder, Van Morrison, Guns & Roses and Rodrigo y Gabriela. Click here for repertoire. Goza has performed multiple times at the Mohegan Sun Wolfden, Hartford Wadsworth Atheneum, Elizabeth Park in West Hartford, River Camelot Cruises, festivals, colleges, libraries and clubs throughout New England. They are listed with many top agencies including James Daniels, Soloman, East West, Landerman, Pyramid, Cutting Edge and have played hundreds of weddings and similar functions. Regular performances in the Hartford area include venues such as: Casona, Chango Rosa, La Tavola Ristorante, Arthur Murray Dance Studio and Elizabeth Park. For more information about GOZA and for our performance schedule, please visit our website at www.gozaband.com or call David Giardina at 860.568-1172. We look forward -
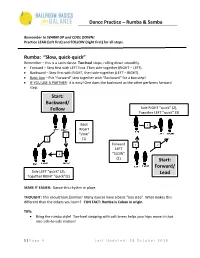
Dance Practice – Rumba & Samba Rumba: “Slow, Quick-Quick” Start
Dance Practice – Rumba & Samba Remember to WARM UP and COOL DOWN! Practice LEAD (left first) and FOLLOW (right first) for all steps. Rumba: “Slow, quick-quick” Remember – this is a Latin dance. Toe-heel steps, rolling down smoothly. • Forward – Step first with LEFT foot. Then side-together (RIGHT – LEFT). • Backward – Step first with RIGHT, then side-together (LEFT – RIGHT). • Basic box – Put “Forward” step together with “Backward” for a box step! • IF YOU USE A PARTNER- it is easy! One does the backward as the other performs forward step. Start: Backward/ Follow Side RIGHT “quick” (2), Together LEFT “quick” (3) Back 3 RIGHT 1 “slow” 2 (1) Forward 1 2 LEFT 3 “SLOW” (1) Start: Forward/ Side LEFT “quick” (2), Lead Together RIGHT “quick”(3) MAKE IT EASIER: Dance this rhythm in place. THOUGHT: This should look familiar! Many dances have a basic “box step”. What makes this different than the others you learn? FUN FACT: Rumba is Cuban in origin. TIPS: • Bring the rumba style! Toe-heel stepping with soft knees helps your hips move in that nice side-to-side motion! 1 | Page 4 Last Updated: 28 October 2018 Dance Practice – Rumba & Samba • Add “Figure 8” hips if you like! In addition to the side-to-side hip motion, allow them to travel forward and backward as you step. (Hip moves forward on the same side as the foot that is stepping.) Samba: “Quick-quick, slow” Remember – this is a Latin dance. If you feel you can, step smoothly toe-heel. • Side-together-back*– – Step side-together (LEFT -RIGHT), then step backward LEFT. -

Brasilian Rhythms and Drumming Techniques
BRASILIAN RHYTHMS AND DRUMMING TECHNIQUES Dr. Jason Koontz Director of Percussion Studies Eastern Kentucky University GENERAL CHARACTERISTICS OF AFRO-BRASILIAN MUSIC *Call and response *Rhythmic complexity (syncopation & polyrhythm) *Structure based on melodic/rhythmic ostinato patterns *Use of timeline/clave *Music as means of communal participation SAMBA - AFRO-BRASILIAN URBAN POPULAR SONG/DANCE FORM Carnival samba (e.g. Samba Batucada and Samba Enredo (Rio,São Paulo), Axé (Bahia) §Characterized by heavy percussion, songs about themes presented in Carnival Pagode (Year-round) samba §Characterized by light percussion and plucked string accompaniment (guitar, cavaquinho) §Songs often satiric, witty, improvised Partido Alto Rhythm Variations A ™2 ≈ ¿™ ¿ ¿ ¿ ¿ ≈ ¿ ¿ ™ / 4 J 3 B ™ ¿ ¿ ≈ ¿ ¿ ≈ ¿™ ¿ ¿ ™ / J 5 C ™ ≈ ¿ ¿ ‰ ¿ ¿ ¿ ¿™ ¿ ™ / J 7 D ™ ≈ ¿ ¿ ‰ ¿ ¿ ¿ ≈ ¿ ¿ ™ / J 9 E *"palma da mão" rhythm ™ ¿™ ¿ ‰ ¿ ¿™ ¿ ‰ ¿ / J J PAGODE INSTRUMENTS: Surdo de Mão – Bass drum instrument played with the hand (a.k.a. Tan Tan, Rebolo) Tamborim (tom-boo-reem), a small single-headed frame drum Pandeiro, (pahn-dey-roo) a tambourine Reco-Reco (hecko-hecko) – scraped metal spring instrument (like a metal Guiro) Cuica (Kwee-Ka) friction drum Cavaquinho – Brasilian counterpart to the Portuguese Cavaquinho, and Ukulele (steel strings G-D-B-G) Pagode (pah-go-jee) rhythms A pattern 1 B pattern 2 > > > > > > > > ° ™2 œ œ œ ™ ™ œ œ œ œ œ œ œ œ ™ Cuíca / ™4 ≈ œ œ œ ≈ œ œ ™ ™ œ œ œ œ œ œ œ œ ™ ™2 ≈ ≈ ™ ™ ≈ ≈ ™ Tamborim / ™4 ¿ ¿ ¿ ¿ ¿ ¿ ¿ ¿ ¿ ™ ™ ¿ ¿ ¿ ¿ ¿ ¿ ¿ ¿ ¿ ™ *"Teleco-teco" rhythm (based on Partido Alto) >. >. >o >. >. >. >o >. ™ o o ™ ™ ™ 2 >¿ >¿ o >¿ ≈ o o ¿ ¿ ¿ ¿ ¿ ¿ ¿ ¿ Pandeiro / ™4 ≈ œ œ œ œ œ ™ ™ œ œ œ œ œ œ œ œ ™ t f h f t f h f t f h f t f h f . -
Apresentação Do Powerpoint
GEÓRGIA EDUCAÇÃO DANÇAS 24/09/2020 SOARES FÍSICA DE SALÃO DANÇA DE SALÃO A dança de salão ou dança social é formada por vários tipos de dança realizados por um casal. Elas são utilizadas como forma de socialização, entretenimento, integração e, até mesmo, competição. 2 Histórico Na Alta Idade Media encontramos as primeiras evidências do substrato que mais tarde vai se conceituar no que entendemos por dança de salão. Neste período, a dança persistiu nas feiras, nos limites dos castelos e nos pequenos burgos nascentes, umas inventadas e outras trazidas do oriente, para onde os dançarinos iam acompanhando os cruzados. Aos poucos, as “danças” foram imitadas pelos nobres e depuradas pelos mestres-de-baile, sendo acrescidas e adaptadas de características pessoais. 3 No século XIX, a dança começou a fazer parte dos encontros da nobreza em seus salões; a dança de salão, denominada genericamente como danças sociais, executada aos pares, em bailes, ou reuniões, deixa de ser considerada coisa de velho e fora de moda, para fazer parte da Educação da aristocracia da época, diferenciando-se da classe pobre que praticava as danças folclóricas. 4 Aprender a dançar com um parceiro parece ir muito além do aprendizado. Mover o corpo em companhia de outro, harmonizando movimentos em sintonia, num mesmo ritmo, resulta em uma união do ser físico numa quase mágica sincronia. Proporciona um encontro consigo mesmo, a partir do encontro com o outro sendo um canal de expressão dos sentimentos por meio dos movimentos. 5 Nos Estados Unidos, surgiram danças com o swing, realizada por negros ao som de jazz, na década de 20. -

New York University in Buenos Aires Class Code: V95.9751002 / K.2094001002 the Multiple Faces of Tango: a Cultural Critique of Identity (English Section)
New York University in Buenos Aires Class Code: V95.9751002 / K.2094001002 The multiple faces of tango: a cultural critique of identity (English Section) Professor: Edgardo Dieleke August/December, 2010 Office hours: To be confirmed Mon and Thurs 3:30pm‐ 5pm Email: [email protected] Classroom: TBA Course Description When tango was born in Buenos Aires, in the second half of the XIX century, Argentina was going through major changes. With the arrival of millions of immigrants, the shape of the city and its society suffered an intense process of modernization. Tango, a product from the bordello considered a threat to national identity, came to be a global success in only twenty years. Acclaimed in Paris and New York, despite its origins, tango became a symbol for Argentina, in a process of negotiations and the control of sexuality and gender and class relations. In this sense, tango reveals itself to be a cultural product that condenses ‐beyond the richness of the music‐ many of the key debates regarding the relationships between culture and society. This course proposes, through the reading of tango lyrics, films and novels, a critical analysis of theoretical problems such as national identity, gender studies and the consumption of culture in a global era. The course will be divided in three parts a) The origins of tango and national identity b) Gender relations: from the forbidden brothels to the movies c) After‐tango: contemporary uses of tango. The course is organized in a format that combines lectures with a seminar‐style class, encouraging class discussions as well as different sights to the city, connected to tango. -

International Dance Conservatory – Ballroom Program
INTERNATIONAL DANCE CONSERVATORY – BALLROOM PROGRAM YEAR FALL SPRING Year 1 Latin Ballroom School Figures Latin Ballroom School Figures (Bronze level) (Silver & Gold levels) Standard Ballroom School Figures Standard Ballroom School Figures (Bronze level) (Silver & Gold levels) Year 2 Latin Technique 1 Latin Technique 2 Latin Ballroom 1 Latin Ballroom 2 Standard Technique 1 Standard Technique 2 Standard Choreography 1 Standard Choreography 2 Year 3 American Smooth 1 American Smooth 2 Student Choreography 1 Student Choreography 2 Advanced Ballroom Technique 1 Advanced Ballroom Technique 2 Year 4 Business of Ballroom Intro to Ballroom Instruction Advanced Choreography 1 Advanced Choreography 2 INTERNATIONAL DANCE CONSERVATORY – BALLROOM PROGRAM Advanced Ballroom Technique 1 & 2 This is an advanced class that focuses on the body mechanics, timing, footwork, partnering, style, expression, and emotion of many Ballroom & Latin dances. Students will continue to develop a deeper understanding of the techniques and stylings of each dance. Students will apply this training in the demonstration of their Latin, Ballroom, and Smooth competition routines. Advanced Choreography 1 & 2 This is an advanced class that focuses on learning open choreography in many Ballroom and Latin Dances. Students will experience this creative process first hand and apply their technique to this choreography. Students will perform these open routines with attention to technical proficiency and embodying the character of each dance. American Smooth 1 & 2 This is an advanced class that focuses on learning open choreography in all four American Smooth Ballroom Dances - Waltz, Tango, Foxtrot, & Viennese Waltz. Students will be prepared to compete in all four dances at the Open Amateur Level. -

Samba Footwork Manual
Samba ! Brief History Samba is a dance and musical genre originating in Brazil, with roots from Africa via the West African slave trade and African religious traditions, particularly Angola and the Congo. Introduced in 1917, the Samba wasn't adopted by Brazil as a ballroom dance until 1930. In Brazil, Samba is mostly danced solo, and remains especially popular during celebrations of the Brazilian Carnival in Rio de Janeiro. The festive mood of the dance is responsible for its continued popularity. Samba is considered to be one of the most popular Brazilian cultural expressions, being is an icon of Brazilian national identity. Samba National Day is celebrated on December 2 every year! Samba has a very specific rhythm, highlighted by characteristic Brazilian musical instruments. To achieve the true character of the Samba, a dancer must give it a happy, flirtatious and exuberant interpretation. Characteristics and Technique Tempo: 80-125 beats per minute Rhythm: 1a2, 3a4 Time signature: 2/4 Principal characteristics of the Samba are the rapid steps taken on a quarter of a beat and the pronounced rocking motion and sway of the dance. The basic footwork of the Samba includes fast, three-step weight changes with a slight knee lift, led with alternating feet. The basic rhythm is ‘quick, quick, slow’. The major action of Samba, the ‘Samba Bounce Action’ gives the dance its unique look and feel. It is a gentle, rhythmic action felt through the knees and ankles. This action should appear effortless and carefree and should never be exaggerated. The ‘Samba Bounce Action’ is quite difficult to master, but really adds to the overall character of the Samba. -

Samba, Improvisation, and Afro-Politics in 1970S Brazil
Bocskay, S. Undesired Presences: Samba, Improvisation, and Afro-politics in 1970s Brazil. Latin American Research Review. 2017; 52(1), pp. 64- 78. DOI: https://doi.org/10.25222/larr.71 LITERATURE AND CULTURAL STUDIES Undesired Presences: Samba, Improvisation, and Afro-politics in 1970s Brazil Stephen Bocskay Universidade Federal de Pernambuco, BR [email protected] This article explores the role of the samba subgenre partido alto as a mode of resistance to modernization and the Brazilian military regime’s disfiguration of samba music in the 1970s. This resistance ultimately led a handful of samba musicians to create the Grêmio Recreativo de Arte Negra Escola de Samba Quilombo in 1975. While it is true that Quilombo nurtured Afro-Brazilian music and culture, the author demonstrates that its leader and cofounder, Antônio Candeia Filho, acted as a samba preservationist and a pioneer, referencing music of the African diaspora, but also as someone who drew the line when it came to espousing Pan-Africanism. The aversion to Pan- Africanism in Rio de Janeiro’s samba community heightened in the late 1970s, as Black Soul, among other foreign sounds and cultural presences, was perceived as a threat to the primacy of samba. Este ensaio estuda o papel do subgênero de samba partido-alto na resistência à modernização e à descaracterização do samba durante o regime militar brasileiro na década de 1970. Tal resistência estimulou um bom número de sambistas a fundar o Grêmio Recreativo de Arte Negra Escola de Samba Quilombo em 1975. Embora o Quilombo tenha alimentado a música e a cultura afro-brasileira, o autor demonstra que seu líder e cofundador, Antônio Candeia Filho, atuou como preservacionista e pioneiro do samba —inspirando-se na música da diáspora africana—, mas também como alguém que estabeleceu limites quando se tratava de desposar o pan-africanismo.