Biodiversity of Orthoptera 10
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Orthoptera Acrididae
Provided for non-commercial research and education use. Not for reproduction, distribution or commercial use. Vol. 12 No. 1 (2019) Egyptian Academic Journal of Biological Sciences is the official English language journal of the Egyptian Society for Biological Sciences, Department of Entomology, Faculty of Sciences Ain Shams University. Entomology Journal publishes original research papers and reviews from any entomological discipline or from directly allied fields in ecology, behavioral biology, physiology, biochemistry, development, genetics, systematics, morphology, evolution, control of insects, arachnids, and general entomology. www.eajbs.eg.net ------------------------------------------------------------------------------------------------------ Citation: Egypt. Acad. J. Biolog. Sci. (A. Entomology) Vol. 12(1) pp: 153- 161 (2019) Egypt. Acad. J. Biolog. Sci., 12(1):153– 161 (2019) Egyptian Academic Journal of Biological Sciences A. Entomology ISSN 1687- 8809 www.eajbs.eg.net Cytogenetic and Meiotic Studies Reveal Conservatism in Acrida turrita (Linnaeus 1758) (Orthoptera: Acrididae) from Lagos, Nigeria Adekoya, K. O1*., Fakorede, S. T1., Okoro, A. N1. and Akpan, U. U1. 1Cell Biology and Genetics Department, University of Lagos, Nigeria E. Mail: [email protected] __________________________________________________________ ARTICLE INFO ABSTRACT Article History The Acrididae exhibits a stable karyotypic uniformity or Received:13/1/2019 conservatism and are a typical specimen for cytological and meiotic Accepted:8/2/2019 investigations. Despite the diversity and cytotaxonomic value of this _______________ family, however there are only a few studies on their karyology. Keywords: This paper is therefore aimed at describing the karyotype and Meiosis, meiotic behaviours of chromosomes of Acrida turrita from Nigeria, Chiasmata, West Africa. Ten (10) male A. turrita grasshoppers were randomly Karyotype, Acrida collected from different locations in the University of Lagos turrita, Nigeria community between May and June, 2018. -

Colonization of a Newly Cleaned Cave by a Camel Cricket: Asian Invasive Or Native?
Lavoie et al. Colonization of a newly cleaned cave by a camel cricket: Asian invasive or native? Kathleen Lavoie1,2, Julia Bordi1,3, Nacy Elwess1,4, Douglas Soroka5, & Michael Burgess1,6 1 Biology Department, State University of New York Plattsburgh, 101 Broad St., Plattsburgh, NY 12901 USA 2 [email protected] (corresponding author) 3 [email protected] 4 [email protected] 5 Greater Allentown Grotto, PA [email protected] 6 [email protected] Key Words: camel crickets, Orthoptera, Rhaphidophoridae, invasive species, recovery of biota, Diestrammena, Diestramima, Crystal Cave, Pennslyvania. Crystal Cave in Kutztown, Pennsylvania, was discovered in 1871 while quarrying for limestone (Stone 1953). Crystal Cave is developed in a belt of Ordovician-age limestone and has an abundance of formations. The cave is about 110 m in extent with an upper level, and access is restricted by a blockhouse (Stone 1953). Crystal Cave is the oldest continually-operating commercial cave in the state, opening for a Grand Illumination in 1872 (Crystal Cave History 2010). It currently hosts about 75,000 visitors a year (K. Campbell, personal communication). Early visitors were guided using candles, oil, and kerosene lanterns, and for a grand lighting, kerosene was spilled onto flowstone and set ablaze to illuminate some of the larger rooms (Snyder 2000). By 1919, the cave was lit with battery-powered lights, and in 1929 5000 feet of wiring with 225 light bulbs was installed. In 1974 new concealed wiring was installed with indirect sealed-beam spotlights (Snyder 2000). Crystal Cave has been heavily impacted by humans, and it showed. Soroka and Lavoie (2017) reported on work to clean up the cave to return it to more natural conditions by removal of soot and grime using power washing and scrubbing. -

A Revision of the Genus Callip Tamus Serville (Orthoptera : Acrididae)
.- e V A REVISION OF THE GENUS CALLIP TAMUS SERVILLE (ORTHOPTERA : ACRIDIDAE) BY N. D. JAGO Univenity of Ghana. Accra Pp. 287-350; 26 Text-jigtcres BULLETIN OF THE BRISISH MUSEUM (NATURAL HISSORY) ENSOMOLOGY Vol. 13 No. 9 LONDON: 1963 THE BULLETIS OF THE BRITISH BIUSEUM (NATURAL HISTORY), institrited in 1949, is isszted iit jizte series, correspondillg to the Departments of the Alirsezm, niid mz Historicnl series. Parts Lcill appear nt irregzrlnr iiitervals as they become retidy. Volimes vil1 coiztaiit aboitt three OY fow htcndred pnges, nitd will not itecessnrily be cmnpleted withilz o.ne cnlenhr year. Tltis paper is Vol. 13, Xo. g of the Entomological series. The nbbreviated titles ojperiodicals cited follou those of tka WorId List of Scientijic Periodicals. 0 Trrictees o[ the British Miiseuni 196.3 PRINTED BY ORDER OF THE TRUSTEES OF THE BRITISH iMUSEUM ~sslldj1 .\I(Z)' 196.3 Price Ti;w+v-tz;.o Sltillings A REVISION OF THE GENUS CALLIP TAMUS SERVILLE (ORTHOPTERA : ACRIDIDAE) By N. D. JAGO CONTENTS Page INTRODUCTION . 289 MATERIAL . 292 TREATMENT 294 ACKXOWLEDGEMENTC. * 294 KEY TO THE GENERA OF THE SUBFAMILY CALLIPTAMINAE . 295 CALLIPTAMUSServille, 1831 . 29s SYNOPSIS The trans-Palaearctic genus Callipíamus Serville is revised, thirteen species now being included in the genus. The genus consists of two main elements, a northern temperate group of four species and a southern ternperate group of nine. The genus Metromerus Uvarov is synonymized with Calliptamus. A provisional key to genera in the sub-family Calliptaminae has been drawn up, together with keys to species and subspecies in the genus Cailiptamus. Observations are given on polymorphism in the genus, geographical vanation, and posible correlation of variation with climatic factors. -

Status and Protection of Globally Threatened Species in the Caucasus
STATUS AND PROTECTION OF GLOBALLY THREATENED SPECIES IN THE CAUCASUS CEPF Biodiversity Investments in the Caucasus Hotspot 2004-2009 Edited by Nugzar Zazanashvili and David Mallon Tbilisi 2009 The contents of this book do not necessarily reflect the views or policies of CEPF, WWF, or their sponsoring organizations. Neither the CEPF, WWF nor any other entities thereof, assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, product or process disclosed in this book. Citation: Zazanashvili, N. and Mallon, D. (Editors) 2009. Status and Protection of Globally Threatened Species in the Caucasus. Tbilisi: CEPF, WWF. Contour Ltd., 232 pp. ISBN 978-9941-0-2203-6 Design and printing Contour Ltd. 8, Kargareteli st., 0164 Tbilisi, Georgia December 2009 The Critical Ecosystem Partnership Fund (CEPF) is a joint initiative of l’Agence Française de Développement, Conservation International, the Global Environment Facility, the Government of Japan, the MacArthur Foundation and the World Bank. This book shows the effort of the Caucasus NGOs, experts, scientific institutions and governmental agencies for conserving globally threatened species in the Caucasus: CEPF investments in the region made it possible for the first time to carry out simultaneous assessments of species’ populations at national and regional scales, setting up strategies and developing action plans for their survival, as well as implementation of some urgent conservation measures. Contents Foreword 7 Acknowledgments 8 Introduction CEPF Investment in the Caucasus Hotspot A. W. Tordoff, N. Zazanashvili, M. Bitsadze, K. Manvelyan, E. Askerov, V. Krever, S. Kalem, B. Avcioglu, S. Galstyan and R. Mnatsekanov 9 The Caucasus Hotspot N. -
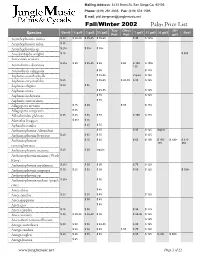
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Saving Tahina — You Can Help
July 2020 NEWSLETTER SAVING TAHINA — YOU CAN HELP PalmTalk (the public forum of the International Palm Society) is initiating a pro- gram to preserve Tahina spectabilis, an endangered palm that PalmTalk brought to the world’s attention in 2007. The discovery of this enormous, new palm gen- erated worldwide interest, but now due to its extremely limited and fragile range in Madagascar, Tahina needs our help. Please visit PalmTalk (go to www. palms.org, and click on the PalmTalk Forums tab) to see how the IPS and the Roy- al Botanic Gardens, Kew are partnering with a local village in Madagascar to help save this amazing palm. You can make a donation to the pro- ject and be a part of this unique partnership in conser- vation. Please donate today! One of the first photos posted on PalmTalk of the mysterious palm that came to be known as Tahina spectabilis and the little girl (at the time) for whom it was named, Anne-Tahina Metz. Volume 8.06 · July 2020 · Newsletter of the International Palm Society | Editor: Andy Hurwitz [email protected] Virtual hiking on Reunion Island, lowlands editionby Andy Hurwitz This article continues the “virtual hike” and tour of Reunion’s palms that began in last month’s News- letter. This month, we explore the palms and landscapes of the low to middle elevations. We begin by traveling to the windward eastern coast of the island, just south of Piton-Sainte-Rose, to ramble in Anse des Cascades. This idyllic cove is situated among groves of palm trees. The park is cool and shady. -

Nansei Islands Biological Diversity Evaluation Project Report 1 Chapter 1
Introduction WWF Japan’s involvement with the Nansei Islands can be traced back to a request in 1982 by Prince Phillip, Duke of Edinburgh. The “World Conservation Strategy”, which was drafted at the time through a collaborative effort by the WWF’s network, the International Union for Conservation of Nature (IUCN), and the United Nations Environment Programme (UNEP), posed the notion that the problems affecting environments were problems that had global implications. Furthermore, the findings presented offered information on precious environments extant throughout the globe and where they were distributed, thereby providing an impetus for people to think about issues relevant to humankind’s harmonious existence with the rest of nature. One of the precious natural environments for Japan given in the “World Conservation Strategy” was the Nansei Islands. The Duke of Edinburgh, who was the President of the WWF at the time (now President Emeritus), naturally sought to promote acts of conservation by those who could see them through most effectively, i.e. pertinent conservation parties in the area, a mandate which naturally fell on the shoulders of WWF Japan with regard to nature conservation activities concerning the Nansei Islands. This marked the beginning of the Nansei Islands initiative of WWF Japan, and ever since, WWF Japan has not only consistently performed globally-relevant environmental studies of particular areas within the Nansei Islands during the 1980’s and 1990’s, but has put pressure on the national and local governments to use the findings of those studies in public policy. Unfortunately, like many other places throughout the world, the deterioration of the natural environments in the Nansei Islands has yet to stop. -

Wild-Harvested Edible Insects
28 Six-legged livestock: edible insect farming, collecting and marketing in Thailand Collecting techniques Wild-harvested edible insects Bamboo caterpillars are mainly collected in the north of Thailand. Apart from farmed edible insects like Bamboo caterpillars were tradi onally crickets and palm weevil larvae, other collected by cutting down entire edible insect species such as silkworm bamboo clumps to harvest the pupae, grasshoppers, weaver ants and caterpillars. This approach was bamboo caterpillars are also popular destruc ve and some mes wasteful food items and can be found in every of bamboo material. More recently a market. less invasive collec on method has been tried. Sustainable collec on Grasshoppers, weaver ants, giant without cutting bamboo trees is water bugs and bamboo caterpillars starting to be practised by local are the most popular wild edible people. Mr.Piyachart, a collector of insects consumed. Grasshoppers are bamboo caterpillars from the wild, collected in the wild, but mainly was interviewed in Chiang Rai Province imported from Cambodia; weaver to learn about his sustainable ants and bamboo caterpillars are collecting method. The adult harvested in the wild seasonally. caterpillar exits, a er pupa emergence, from a hole at the base of the bamboo stem. The fi rst or second internode is Bamboo caterpillar examined to reveal the damage (Omphisa fuscidenƩ alis caused by the bamboo caterpillar and Hampson, Family its loca on. The denseness of an Pyralidae) internode is a clue to indicate the presence of bamboo caterpillars. The Known in Thai as rod fai duan or ‘the harves ng of bamboo caterpillars is express train’ the larvae live inside conducted by slicing the specifi c bamboo plants for around ten months. -

2951 – 3000 Trinidad to British Guiana
2951 – 3000 Trinidad to British Guiana [Inside cover] Book 9 Acanthophoenix 2956 Lecythis 2963 Acrocomia 2961 Licuala 2978 Ananas 2993 Livistona 2982 Archontophoenix 2983 Lodoicea 2985 Areca 2953 2953 Mauritia 2984 “ 2954 Mokka-mokka 2997 Astrocaryum 2957 Nipa 2981 “ 2986 Pachira 2976 “ 2987 “ 3000 Asystasia 2964 Passiflora 2952 Bauhinia 2960 “ 2995 Borassus 2979 Peltogyne 2970 Bromelia 2996 Pithecolobium 2965 Caryocar 2999 Randia 2994 Citrus 2998 Samanea 2966 Copernicia 2977 Undetermined 2967 Crotolaria 2973 “ In ink:]Ochna 2971 “ 2974 “ 2972 Desmoncus 2951 “ 2989 Diospyrus 2968 “ 2990 Euterpe 2955 “ 2991 Gmelina 2969 “ 2992 Hyphaene 2980 Zingerberaciae 2958 Ixora 2975 TILLANDSIA 2996 Jacaranda 2962 2951 Demoncus minor? See Harold Loomis’ notes and photo of inflorescence. #54 Villainously spacing & hooked climbing palm reminding one of those terrible Rattan palms of the Orient. When in fruit its bunches of deep scarlet fruits are attractive. A denizen of the deep shade forest and requiring moisture. From my experience in Coconut Grove with this genus I judge it wants half shade. Collected in Arena Forest Reserve Trinidad. 2/4/32 [In pencil] Loomis Photo 220 See D. F. Photo 18454-5 2952 P. H. Dorsett [In ink] “rubra?” Passiflora Sp Wild species of passion vine collected in the outskirts of the town of Eleuthera Bluff Island of Eleuthera Bahamas. Attractive looking species useful [Break in text, in ink] 1 single plant in pot Dec. 14/32. C. F. [Continuation of text] for breeding purposes. 1-7-32 (I’d like a few seeds or plants for my passiflora collection in Coconut Grove) Passiflora sp. Wild species growing in pothole in rocky soil of Eleuthera Bluff a colored town on Eleuthera Island. -

(Orthoptera, Caelifera, Acrididae) on the Subfamily Level Using Molecular Markers
e-ISSN 1734-9168 Folia Biologica (Kraków), vol. 67 (2019), No 3 http://www.isez.pan.krakow.pl/en/folia-biologica.html https://doi.org/10.3409/fb_67-3.12 The Evaluation of Genetic Relationships within Acridid Grasshoppers (Orthoptera, Caelifera, Acrididae) on the Subfamily Level Using Molecular Markers Igor SUKHIKH , Kirill USTYANTSEV , Alexander BUGROV, Michael SERGEEV, Victor FET, and Alexander BLINOV Accepted August 20, 2019 Published online September 11, 2019 Issue online September 30, 2019 Original article SUKHIKH I., USTYANTSEV K., BUGROV A., SERGEEV M., FET V., BLINOV A. 2019. The evaluation of genetic relationships within Acridid grasshoppers (Orthoptera, Caelifera, Acrididae) on the subfamily level using molecular markers. Folia Biologica (Kraków) 67: 119-126. Over the last few decades, molecular markers have been extensively used to study phylogeny, population dynamics, and genome mapping in insects and other taxa. Phylogenetic methods using DNA markers are inexpensive, fast and simple to use, and may help greatly to resolve phylogenetic relationships in groups with problematic taxonomy. However, different markers have various levels of phylogenetic resolution, and it’s important to choose the right set of molecular markers for a studied taxonomy level. Acrididae is the most diverse family of grasshoppers. Many attempts to resolve the phylogenetic relationships within it did not result in a clear picture, partially because of the limited number of molecular markers used. We have tested a phylogenetic resolution of three sets of the most commonly utilized mitochondrial molecular markers available for Acrididae sequences in the database: (i) complete protein-coding mitochondrial sequences, (ii) concatenated mitochondrial genes COI, COII, and Cytb, and (iii) concatenated mitochondrial genes COI and COII. -

N-P Co-Limitation of Primary Production and Response of Arthropods to N and P in Early Primary Succession on Mount St. Helens Volcano
SUNY Geneseo KnightScholar Biology Faculty/Staff Works Department of Biology 2010 N-P co-limitation of primary production and response of arthropods to N and P in early primary succession on Mount St. Helens Volcano John G. Bishop Niamh B. O'Hara Jonathan H. Titus Jennifer L. Apple SUNY Geneseo Richard A. Gill See next page for additional authors Follow this and additional works at: https://knightscholar.geneseo.edu/biology This work is licensed under a Creative Commons Attribution 4.0 License. Recommended Citation Bishop J.G., O'Hara N.B., Titus J.H., Apple J.L., Gill R.A., Wynn L. (2010) N-P co-limitation of primary production and response of arthropods to N and P in early primary succession on Mount St. Helens Volcano. PLoS ONE 5: -. doi: 10.1371/journal.pone.0013598 This Article is brought to you for free and open access by the Department of Biology at KnightScholar. It has been accepted for inclusion in Biology Faculty/Staff Works by an authorized administrator of KnightScholar. For more information, please contact [email protected]. Authors John G. Bishop, Niamh B. O'Hara, Jonathan H. Titus, Jennifer L. Apple, Richard A. Gill, and Louise Wynn This article is available at KnightScholar: https://knightscholar.geneseo.edu/biology/2 N-P Co-Limitation of Primary Production and Response of Arthropods to N and P in Early Primary Succession on Mount St. Helens Volcano John G. Bishop1*, Niamh B. O’Hara1¤a, Jonathan H. Titus2, Jennifer L. Apple1¤b, Richard A. Gill3, Louise Wynn1 1 School of Biological Sciences, Washington State University, Vancouver, Washington, United States of America, 2 Department of Biology, SUNY Fredonia, Fredonia, New York, United States of America, 3 Brigham Young University, Provo, Utah, United States of America Abstract Background: The effect of low nutrient availability on plant-consumer interactions during early succession is poorly understood. -

(Orthoptera) and Their Phylogenetic Implications Within Tetrigoidea
Mitochondrial genomes of eight Scelimeninae species (Orthoptera) and their phylogenetic implications within Tetrigoidea Ran Li1, Xiaoli Ying1, Weian Deng2, Wantao Rong2 and Xiaodong Li2 1 College of Life Sciences, Nanjing Normal University, Nanjing, China 2 School of Chemistry and Bioengineering, Hechi University, Yizhou, China ABSTRACT Scelimeninae is a key member of the pygmy grasshopper community, and an important ecological indicator. No mitochondrial genomes of Scelimeninae have been reported to date, and the monophyly of Scelimeninae and its phylogenetic relationship within Tetrigidae is still unclear. We sequenced and analyzed eight nearly complete mitochondrial genomes representing eight genera of Scelimeninae. These mitogenomes ranged in size from 13,112 to 16,380 bp and the order of tRNA genes between COII and ATP8 was reversed compared with the ancestral order of insects. The protein-coding genes (PCGs) of tetrigid species mainly with the typical ATN codons and most terminated with complete (TAA or TAG) stop codons. Analyses of pairwise genetic distances showed that ATP8 was the least conserved gene within Tetrigidae, while COI was the most conserved. The longest intergenic spacer (IGS) region in the mitogenomes was always found between tRNASer(UCN) and ND1. Additionally, tandem repeat units were identified in the longest IGS of three mitogenomes. Maximum likelihood (ML) and Bayesian Inference (BI) analyses based on the two datasets supported the monophyly of Tetriginae. Scelimeninae was classified as a non-monophyletic subfamily.