New Bulk Sulfur Measurements of Martian Meteorites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shergotty Basalt, 5 Kg Seen to Fall Introduction the Shergotty Achondrite Fell on August 25, 1865 at 9:00 A.M
V. Shergotty basalt, 5 kg seen to fall Introduction The Shergotty achondrite fell on August 25, 1865 at 9:00 a.m. near a town called Shergahti in Bihar State, India after detonations were heard (Graham et al. 1985). Duke (1968) refers to several stones with fusion crusts, but this has not been confirmed. The main mass is at the Museum of the Geological Survey in Calcutta, India (figure V-1). In 1984, an international consortium was organized by J. C. Laul to study ~30 grams of Shergotty in detail (Laul 1986a, b). Shergottites (Shergotty, Zagami, EETA79001B, QUE94201, Los Angeles) are texturally and mineralogically similar to terrestrial diabases (although all of the plagioclase has been shocked to maskelynite), but quite distinct petrologically and chemically from the rest of the basaltic achondrites (Stolper et al. 1979). Stolper and McSween (1979) and others have noted that Shergotty crystallized under relatively oxidizing conditions. Figure V-1. Photograph of Shergotty meteorite The Shergotty meteorite has been severely shocked and showing fusion crust and borken surfaces. Two saw is considered the “type locality” for maskelynite (dense cuts are visible. Sample is about 25 cm across. Photo plagioclase glass). In fact, it has proven to be very kindly provided by Prof. N. Bhandari, Director, Physical Research Laboratory, Ahmedabad, India. difficult to date the original crystallization event of Figure V-2. Photograph of slab of Shergotty meteorite showing basaltic texture and alignment of pyroxene crystals. Note the inclusion of black glass in the center of this slab. This is figure 1 in Duke (1968). Photo courtesy of U. -
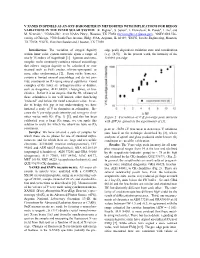
V Xanes in Spinels As an Oxy-Barometer in Meteorites with Implications for Redox Variations in the Inner Solar System
V XANES IN SPINELS AS AN OXY-BAROMETER IN METEORITES WITH IMPLICATIONS FOR REDOX VARIATIONS IN THE INNER SOLAR SYSTEM. K. Righter1, S. Sutton2, L. Danielson3, K. Pando4, L. Le3, and M. Newville2. 1NASA-JSC, 2101 NASA Pkwy., Houston, TX 77058 ([email protected]), 2GSECARS Uni- versity of Chicago, 9700 South Cass Avenue, Bldg. 434A, Argonne, IL 60439; 3ESCG, Jacobs Engineering, Houston, TX 77058; 4ESCG, Hamilton Sundstrand, Houston, TX 77058 Introduction: The variation of oxygen fugacity edge peak) depend on oxidation state and coordination within inner solar system materials spans a range of (e.g., [4,5]). In the present work, the intensity of the nearly 15 orders of magnitiude [1]. Igneous and meta- XANES pre-edge morphic rocks commonly contain a mineral assemblage that allows oxygen fugacity to be calculated or con- strained such as FeTi oxides, olivine-opx-spinel, or some other oxybarometer [2]. Some rocks, however, contain a limited mineral assemblage and do not pro- vide constraints on fO2 using mineral equilibria. Good examples of the latter are orthopyroxenites or dunites, such as diogenites, ALH 84001, chassignites, or bra- chinites. In fact it is no surprise that the fO2 of many of these achondrites is not well known, other than being "reduced" and below the metal saturation value. In or- der to bridge this gap in our understanding, we have initiated a study of V in chromites in achondrite. Be- cause the V pre-edge peak intensity and energy in chro- mites varies with fO2 (Fig. 1) [3], and this has been Figure 1: Correlation of V K pre-edge peak intensity calibrated over a large fO2 range, we can apply this with IW for spinels in the experiments of [3]. -

Physical Properties of Martian Meteorites: Porosity and Density Measurements
Meteoritics & Planetary Science 42, Nr 12, 2043–2054 (2007) Abstract available online at http://meteoritics.org Physical properties of Martian meteorites: Porosity and density measurements Ian M. COULSON1, 2*, Martin BEECH3, and Wenshuang NIE3 1Solid Earth Studies Laboratory (SESL), Department of Geology, University of Regina, Regina, Saskatchewan S4S 0A2, Canada 2Institut für Geowissenschaften, Universität Tübingen, 72074 Tübingen, Germany 3Campion College, University of Regina, Regina, Saskatchewan S4S 0A2, Canada *Corresponding author. E-mail: [email protected] (Received 11 September 2006; revision accepted 06 June 2007) Abstract–Martian meteorites are fragments of the Martian crust. These samples represent igneous rocks, much like basalt. As such, many laboratory techniques designed for the study of Earth materials have been applied to these meteorites. Despite numerous studies of Martian meteorites, little data exists on their basic structural characteristics, such as porosity or density, information that is important in interpreting their origin, shock modification, and cosmic ray exposure history. Analysis of these meteorites provides both insight into the various lithologies present as well as the impact history of the planet’s surface. We present new data relating to the physical characteristics of twelve Martian meteorites. Porosity was determined via a combination of scanning electron microscope (SEM) imagery/image analysis and helium pycnometry, coupled with a modified Archimedean method for bulk density measurements. Our results show a range in porosity and density values and that porosity tends to increase toward the edge of the sample. Preliminary interpretation of the data demonstrates good agreement between porosity measured at 100× and 300× magnification for the shergottite group, while others exhibit more variability. -

Aqueous Alteration in Martian Meteorites: Comparing Mineral Relations in Igneous-Rock Weathering of Martian Meteorites and in the Sedimentary Cycle of Mars
AQUEOUS ALTERATION IN MARTIAN METEORITES: COMPARING MINERAL RELATIONS IN IGNEOUS-ROCK WEATHERING OF MARTIAN METEORITES AND IN THE SEDIMENTARY CYCLE OF MARS MICHAEL A. VELBEL Department of Geological Sciences, 206 Natural Science Building, Michigan State University, East Lansing, Michigan 48824-1115 USA e-mail: [email protected] ABSTRACT: Many of the minerals observed or inferred to occur in the sediments and sedimentary rocks of Mars, from a variety of Mars-mission spacecraft data, also occur in Martian meteorites. Even Martian meteorites recovered after some exposure to terrestrial weathering can preserve preterrestrial evaporite minerals and useful information about aqueous alteration on Mars, but the textures and textural contexts of such minerals must be examined carefully to distinguish preterrestrial evaporite minerals from occurrences of similar minerals redistributed or formed by terrestrial processes. Textural analysis using terrestrial microscopy provides strong and compelling evidence for preterrestrial aqueous alteration products in a numberof Martian meteorites. Occurrences of corroded primary rock-forming minerals and alteration products in meteorites from Mars cover a range of ages of mineral–water interaction, from ca. 3.9 Ga (approximately mid-Noachian), through one or more episodes after ca. 1.3 Ga (approximately mid–late Amazonian), through the last half billion years (late Amazonian alteration in young shergottites), to quite recent. These occurrences record broadly similar aqueous corrosion processes and formation of soluble weathering products over a broad range of times in the paleoenvironmental history of the surface of Mars. Many of the same minerals (smectite-group clay minerals, Ca-sulfates, Mg-sulfates, and the K-Fe–sulfate jarosite) have been identified both in the Martian meteorites and from remote sensing of the Martian surface. -

Meteorite Collections: Sample List
Meteorite Collections: Sample List Institute of Meteoritics Department of Earth and Planetary Sciences University of New Mexico October 01, 2021 Institute of Meteoritics Meteorite Collection The IOM meteorite collection includes samples from approximately 600 different meteorites, representative of most meteorite types. The last printed copy of the collection's Catalog was published in 1990. We will no longer publish a printed catalog, but instead have produced this web-based Online Catalog, which presents the current catalog in searchable and downloadable forms. The database will be updated periodically. The date on the front page of this version of the catalog is the date that it was downloaded from the worldwide web. The catalog website is: Although we have made every effort to avoid inaccuracies, the database may still contain errors. Please contact the collection's Curator, Dr. Rhian Jones, ([email protected]) if you have any questions or comments. Cover photos: Top left: Thin section photomicrograph of the martian shergottite, Zagami (crossed nicols). Brightly colored crystals are pyroxene; black material is maskelynite (a form of plagioclase feldspar that has been rendered amorphous by high shock pressures). Photo is 1.5 mm across. (Photo by R. Jones.) Top right: The Pasamonte, New Mexico, eucrite (basalt). This individual stone is covered with shiny black fusion crust that formed as the stone fell through the earth's atmosphere. Photo is 8 cm across. (Photo by K. Nicols.) Bottom left: The Dora, New Mexico, pallasite. Orange crystals of olivine are set in a matrix of iron, nickel metal. Photo is 10 cm across. (Photo by K. -

The Nakhlite Meteorites: Augite-Rich Igneous Rocks from Mars ARTICLE
ARTICLE IN PRESS Chemie der Erde 65 (2005) 203–270 www.elsevier.de/chemer INVITED REVIEW The nakhlite meteorites: Augite-rich igneous rocks from Mars Allan H. Treiman Lunar and Planetary Institute, 3600 Bay Area Boulevard, Houston, TX 77058-1113, USA Received 22 October 2004; accepted 18 January 2005 Abstract The seven nakhlite meteorites are augite-rich igneous rocks that formed in flows or shallow intrusions of basaltic magma on Mars. They consist of euhedral to subhedral crystals of augite and olivine (to 1 cm long) in fine-grained mesostases. The augite crystals have homogeneous cores of Mg0 ¼ 63% and rims that are normally zoned to iron enrichment. The core–rim zoning is cut by iron-enriched zones along fractures and is replaced locally by ferroan low-Ca pyroxene. The core compositions of the olivines vary inversely with the steepness of their rim zoning – sharp rim zoning goes with the most magnesian cores (Mg0 ¼ 42%), homogeneous olivines are the most ferroan. The olivine and augite crystals contain multiphase inclusions representing trapped magma. Among the olivine and augite crystals is mesostasis, composed principally of plagioclase and/or glass, with euhedra of titanomagnetite and many minor minerals. Olivine and mesostasis glass are partially replaced by veinlets and patches of iddingsite, a mixture of smectite clays, iron oxy-hydroxides and carbonate minerals. In the mesostasis are rare patches of a salt alteration assemblage: halite, siderite, and anhydrite/ gypsum. The nakhlites are little shocked, but have been affected chemically and biologically by their residence on Earth. Differences among the chemical compositions of the nakhlites can be ascribed mostly to different proportions of augite, olivine, and mesostasis. -

INASA-CB-176518) MAGNETISM Or NAKHLITES and N86-20277 CHASS IGNITES Piaal Technical Keport, 1 .Mar
. 4 FINAL TECHNICAL REPORT PRINCIPAL INVESTIGATOR: Stanley M. Cisowski Department of Geological Sciences University of California Santa Barbara, California 93106 GRANT TITLE: Magnetism of Nakhlites and Chassignites GRANT NUMBER: NASA NAG 9-72 PERIOD: 3/1/84-2/28/85 INASA-CB-176518) MAGNETISM or NAKHLITES AND N86-20277 CHASS IGNITES Piaal Technical Keport, 1 .Mar. 1984 - 28 ?eb. 1965 (California Univ.), 27 p* 3C A03/MF A01 CSCL 03B G3/90 Uncla05UU2s ORIGINAL PAGE IS POOR QUAirfY Through the support of this grant, complete magnetic analyses have been completed for the shergottite meteorites Shergotty, Zagami, EETA 79001, and ALHA 77005, and for nakhlite meteorites Nakhla and Governador Valadares. The studied samples of Shergotty meteorite included the subsamples of the Shergotty Consortium, which were made available for interdisciplinary studies by the Geological Survey of India. Magnetic measurements included high' field hysteresis loops to determine the size of the magnetic grains, thermomagnetic curves to determine the composition of these grains, and remanence measurements to determine the nature of the magnetization that these various samples carry. A detailed paleointensity experiment was conducted on a subsample of Shergotty meteorite to determine the strength of. the magnetic field in which the meteorite was magneti zed. The results of these analyses are that the magnetic carriers for the shergotite and nakhlite meteorites are generally •fine graine?d t i tanamagneti tes similar in composition to the t i tanornagneti tes in oceanic basalts. Although the Shergotty and Zagami meteorites display large variations in remanence intensity, it is believed that the strong magnetisations result from post-sampling contamination in stray magnetic fields. -

Meteorites: an Overview
Meteorites: An Overview Edward R. D. Scott* 1811-5209/11/0007-0047$2.50 DOI: 10.2113/gselements.7.1.47 eteorites come from numerous parent bodies with a wide variety chondrites are the most pristine, of geological histories. A few (~0.5%) come from Mars or the Moon; type 6 are the most metamor- phosed, and type 1 are the most Mthe rest are impact debris from collisions between asteroids orbiting aqueously altered. between Mars and Jupiter. Unlike terrestrial, Martian, and lunar rocks, the Asteroids that melted supply us asteroidal meteorites contain minerals that formed before the Sun and the with three major classes of mete- Solar System, during the growth of planetesimals and planets from the disk orites: irons, which are Fe–Ni of dust and gas around the Sun (“the solar nebula”), and during the first samples from the cores of aster- oids; metal-free silicate rocks from half-billion years of Solar System evolution. asteroidal mantles and crusts, Meteorites from unmelted asteroids are called chondrites; which are called achondrites as they are cosmic sediments composed of particles that were they lack chondrules; and stony irons, which are impact- generated mixtures of achondrites and Fe–Ni metal (Fig. present in the solar nebula (Fig. 1). The most abundant particles are millimeter-sized objects called chondrules, 2). Most irons (85%) are divided into thirteen groups which are solidified droplets of silicate magma. The chon- (called IAB, IIAB, IIC, etc.), which come from separate drules, associated grains of Fe–Ni metal, and a few volume parent bodies. The remaining ~15% probably come from percent or less of calcium–aluminum-rich inclusions another 50-odd parent bodies. -

Reviewing Martian Atmospheric Noble Gas Measurements: from Martian Meteorites to Mars Missions
geosciences Review Reviewing Martian Atmospheric Noble Gas Measurements: From Martian Meteorites to Mars Missions Thomas Smith 1,* , P. M. Ranjith 1, Huaiyu He 1,2,3,* and Rixiang Zhu 1,2,3 1 State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, 19 Beitucheng Western Road, Box 9825, Beijing 100029, China; [email protected] (P.M.R.); [email protected] (R.Z.) 2 Institutions of Earth Science, Chinese Academy of Sciences, Beijing 100029, China 3 College of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100049, China * Correspondence: [email protected] (T.S.); [email protected] (H.H.) Received: 10 September 2020; Accepted: 4 November 2020; Published: 6 November 2020 Abstract: Martian meteorites are the only samples from Mars available for extensive studies in laboratories on Earth. Among the various unresolved science questions, the question of the Martian atmospheric composition, distribution, and evolution over geological time still is of high concern for the scientific community. Recent successful space missions to Mars have particularly strengthened our understanding of the loss of the primary Martian atmosphere. Noble gases are commonly used in geochemistry and cosmochemistry as tools to better unravel the properties or exchange mechanisms associated with different isotopic reservoirs in the Earth or in different planetary bodies. The relatively low abundance and chemical inertness of noble gases enable their distributions and, consequently, transfer mechanisms to be determined. In this review, we first summarize the various in situ and laboratory techniques on Mars and in Martian meteorites, respectively, for measuring noble gas abundances and isotopic ratios. -

(2000) Forging Asteroid-Meteorite Relationships Through Reflectance
Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. B.S. Physics Rensselaer Polytechnic Institute, 1988 M.S. Geology and Planetary Science University of Pittsburgh, 1991 SUBMITTED TO THE DEPARTMENT OF EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN PLANETARY SCIENCES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY FEBRUARY 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: Department of Earth, Atmospheric, and Planetary Sciences December 30, 1999 Certified by: Richard P. Binzel Professor of Earth, Atmospheric, and Planetary Sciences Thesis Supervisor Accepted by: Ronald G. Prinn MASSACHUSES INSTMUTE Professor of Earth, Atmospheric, and Planetary Sciences Department Head JA N 0 1 2000 ARCHIVES LIBRARIES I 3 Forging Asteroid-Meteorite Relationships through Reflectance Spectroscopy by Thomas H. Burbine Jr. Submitted to the Department of Earth, Atmospheric, and Planetary Sciences on December 30, 1999 in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Planetary Sciences ABSTRACT Near-infrared spectra (-0.90 to ~1.65 microns) were obtained for 196 main-belt and near-Earth asteroids to determine plausible meteorite parent bodies. These spectra, when coupled with previously obtained visible data, allow for a better determination of asteroid mineralogies. Over half of the observed objects have estimated diameters less than 20 k-m. Many important results were obtained concerning the compositional structure of the asteroid belt. A number of small objects near asteroid 4 Vesta were found to have near-infrared spectra similar to the eucrite and howardite meteorites, which are believed to be derived from Vesta. -

Extra-Terrestrial Igneous Granites and Related Rocks: a Review of Their Occurrence and Petrogenesis
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright Author's personal copy Lithos 153 (2012) 3–24 Contents lists available at SciVerse ScienceDirect Lithos journal homepage: www.elsevier.com/locate/lithos Extra-terrestrial igneous granites and related rocks: A review of their occurrence and petrogenesis Bernard Bonin ⁎ UMR8148 ‘IDES’, CNRS, Département des Sciences de la Terre, Université de Paris-Sud, F-91405 ORSAY CEDEX, France article info abstract Article history: The telluric planets and the asteroid belt display the same internal structure with a metallic inner core and a Received 4 November 2011 silicate outer shell. Experimental data and petrological evidence in silicate systems show that granite can be Accepted 4 April 2012 produced by extreme igneous differentiation through various types of igneous processes. Available online 14 April 2012 On Moon, 4.4–3.9 Ga granite clasts display dry mineral assemblages. They correspond to at least 8 discrete intru- sive events. Large K/Ca enrichment and low REE abundances in granite relative to KREEP are consistent with sil- Keywords: icate liquid immiscibility, a process observed in melt inclusions within olivine of lunar basalts and in lunar Planetary granites fi A-type meteorites. -

List G - Meteorites - Alphabetical List
LIST G - METEORITES - ALPHABETICAL LIST Specific name Group name Specific name Group name Abee Meteorite EH chondrites EETA 79001 Elephant Moraine Meteorites Acapulco Meteorite acapulcoite and shergottite acapulcoite stony meteorites Efremovka Meteorite CV chondrites Acfer Meteorites meteorites EH chondrites enstatite chondrites achondrites stony meteorites EL chondrites enstatite chondrites ALH 84001 Allan Hills Meteorites and Elephant Moraine meteorites achondrites Meteorites ALHA 77005 Allan Hills Meteorites and enstatite chondrites chondrites shergottite eucrite achondrites ALHA 77307 Allan Hills Meteorites and Fayetteville Meteorite H chondrites CO chondrites Frontier Mountain meteorites ALHA 81005 Allan Hills Meteorites and Meteorites achondrites Gibeon Meteorite octahedrite Allan Hills Meteorites meteorites GRA 95209 Graves Nunataks Meteorites Allende Meteorite CV chondrites and lodranite angrite achondrites Graves Nunataks meteorites Ashmore Meteorite H chondrites Meteorites Asuka Meteorites meteorites H chondrites ordinary chondrites ataxite iron meteorites Hammadah al Hamra meteorites aubrite achondrites Meteorites Barwell Meteorite L chondrites Haveroe Meteorite ureilite Baszkowka Meteorite L chondrites Haviland Meteorite H chondrites Belgica Meteorites meteorites HED meteorites achondrites Bencubbin Meteorite chondrites Hedjaz Meteorite L chondrites Bishunpur Meteorite LL chondrites Henbury Meteorite octahedrite Bjurbole Meteorite L chondrites hexahedrite iron meteorites Brenham Meteorite pallasite HL chondrites ordinary chondrites