Bivalve Mollusk Paleoecology: Trophic and Environmental
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Community-Defined Research Priorities
Journal Pre-proof Fundamental questions and applications of sclerochronology: Community-defined research priorities Tamara Trofimova, Stella J. Alexandroff, Madelyn Mette, Elizabeth Tray, Paul G. Butler, Steven Campana, Elizabeth Harper, Andrew L.A. Johnson, John R. Morrongiello, Melita Peharda, Bernd R. Schöne, Carin Andersson, C. Fred T. Andrus, Bryan A. Black, Meghan Burchell, Michael L. Carroll, Kristine L. DeLong, Bronwyn M. Gillanders, Peter Grønkjær, Daniel Killam, Amy L. Prendergast, David J. Reynolds, James D. Scourse, Kotaro Shirai, Julien Thébault, Clive Trueman, Niels de Winter PII: S0272-7714(20)30708-3 DOI: https://doi.org/10.1016/j.ecss.2020.106977 Reference: YECSS 106977 To appear in: Estuarine, Coastal and Shelf Science Received Date: 1 February 2020 Revised Date: 15 July 2020 Accepted Date: 4 August 2020 Please cite this article as: Trofimova, T., Alexandroff, S.J., Mette, M., Tray, E., Butler, P.G., Campana, S., Harper, E., Johnson, A.L.A., Morrongiello, J.R., Peharda, M., Schöne, B.R., Andersson, C., Andrus, C.F.T., Black, B.A., Burchell, M., Carroll, M.L., DeLong, K.L., Gillanders, B.M., Grønkjær, P., Killam, D., Prendergast, A.L., Reynolds, D.J., Scourse, J.D., Shirai, K., Thébault, J., Trueman, C., de Winter, N., Fundamental questions and applications of sclerochronology: Community-defined research priorities, Estuarine, Coastal and Shelf Science (2020), doi: https://doi.org/10.1016/j.ecss.2020.106977. This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. -

Jocelyn A. Sessa
Jocelyn A. Sessa Current: Assistant Curator of Invertebrate Paleontology, Academy of Natural Sciences, & Assistant Professor, Department of Biodiversity, Earth & Environmental Science of Drexel University. Past Positions: 2016 to 2017 Senior Scientist in Paleontology & Education, American Museum of Natural History. Postdoctoral Fellowships: 2012 to 2016 Departments of Paleontology & Education, American Museum of Natural History. 2010 to 2012 Department of Paleobiology, Smithsonian National Museum of Natural History. 2009 to 2010 Department of Earth Sciences, Syracuse University. Education: Ph.D., 2009 Department of Geosciences, Pennsylvania State University, University Park, PA. M.S., 2003 Department of Geology, University of Cincinnati, Cincinnati, Ohio. B.A., 2000 Department of Geological Sciences, State University of New York at Geneseo, Geneseo, NY. Cum laude, minor in Environmental Studies. Publications (* indicates student author; for student work, ‡ indicates corresponding author): Buczek, A.J.*, Hendy, A., Hopkins, M. Sessa, J.A.‡ 2020. On the reconciliation of biostratigraphy and strontium isotope stratigraphy of three southern Californian Plio-Pleistocene formations. Geological Society of America Bulletin 132 ; doi.org/10.1130/B35488.1. Oakes, R.L., Sessa, J.A. 2020. Determining how biotic and abiotic variables affect the shell condition and parameters of Heliconoides inflatus pteropods from a sediment trap in the Cariaco Basin. Biogeosciences 17:1975–1990; doi.org/10.5194/bg-17-1975-2020. Oakes, R.L., Hill Chase, M., Siddall, M.E., Sessa, J.A. 2020. Testing the impact of two key scan parameters on the quality and repeatability of measurements from CT scan data. Palaeontologia Electronica 23(1):a07; doi.org/10.26879/942. Ferguson, K.*, MacLeod, K.G.‡, Landman, N.H., Sessa, J.A.‡ 2019. -

Geochemical and Microstructural Signals in Giant Clam Tridacna Maxima Recorded Typhoon Events at Okinotori Island, Japan
Geochemical and Microstructural Signals in Giant Clam Tridacna maxima Recorded Typhoon Events at Okinotori Title Island, Japan Author(s) Komagoe, Taro; Watanabe, Tsuyoshi; Shirai, Kotaro; Yamazaki, Atsuko; Uematu, Mitsuo Journal of geophysical research biogeosciences, 123(5), 1460-1474 Citation https://doi.org/10.1029/2017JG004082 Issue Date 2018-05 Doc URL http://hdl.handle.net/2115/71789 Rights Copyright 2018 American Geophysical Union Type article File Information Komagoe_et_al-2018-Journal_of_Geophysical_Research%3A_Biogeosciences.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Journal of Geophysical Research: Biogeosciences RESEARCH ARTICLE Geochemical and Microstructural Signals in Giant Clam 10.1029/2017JG004082 Tridacna maxima Recorded Typhoon Events Key Points: at Okinotori Island, Japan • Tridacna maxima in Okinotori Island made daily growth increments, Taro Komagoe1,2 , Tsuyoshi Watanabe1,2 , Kotaro Shirai3 , Atsuko Yamazaki1,2,4 , and increment-based chronology 3 provided dates for geochemical and Mitsuo Uematu analysis data 18 1Department of Natural History Sciences, Faculty of Science, Hokkaido University, Sapporo, Japan, 2KIKAI institute for Coral • δ Oshell reflected annual SST fluctuations in Okinotori Island Reef Sciences, Kikai town, Japan, 3Atmosphere and Ocean Research Institute, The University of Tokyo, Kashiwa, Japan, • The decrease in increment thickness 4Department of Earth and Planetary Sciences, Faculty of Science, Kyushu University, Fukuoka, Japan and positive peaks in the shell Ba/Ca 18 ratio and δ Oshell corresponded to fall season typhoon approaches to the Abstract To validate the usability of the giant clam shell as a recorder of short-term environmental island changes such as typhoons, we collected a live Tridacna maxima from Okinotori Island, Japan, on 15 June 18 13 2006. -

Active Research Grants
Linda C. Ivany Professor Department of Earth and Environmental Sciences Heroy Geology Laboratory,Syracuse University, Syracuse, NY 13244 phone: (315) 443-3626 / fax: (315) 443-3363 / email: [email protected] http://thecollege.syr.edu/people/faculty/pages/ear/Ivany-Linda.html https://orcid.org/0000-0002-4692-3455 Education Ph.D. in Earth and Planetary Sciences, 1997, Harvard University Advisor: Stephen Jay Gould M.S. in Geology, minor in Zoology, 1990, University of Florida-Gainesville Advisor: Douglas S. Jones B.S. in Geology, minor in Zoology, 1988, Syracuse University Advisor: Cathryn R. Newton Academic Positions 2012-present Professor of Earth Sciences, Syracuse University 2005-2012 Associate Professor of Earth Sciences, Syracuse University 2001-2005 Assistant Professor of Earth Sciences, Syracuse University 2000-2001 Visiting Assistant Professor of Earth Sciences, Syracuse University 1997-2000 Michigan Society Fellow and Visiting Assistant Professor of Geological Sciences, University of Michigan General Research Interests Evolutionary Paleoecology, Paleoclimatology, Stable Isotopes in Paleobiology I am a marine paleoecologist and paleoclimatologist. My interests lie broadly in the evolution of the Earth-life system and how ecosystems and their component taxa evolve and respond to changes in the physical environment. Specific areas of interest include biotic and climatic change during the Paleogene (~65-24 million years ago); use of geochemical data, particularly stable isotopes, derived from accretionary biogenic materials for inference -

Geoscience Department of Geography & Geology Program Review 2007-2014
University of North Carolina Wilmington Master of Science in Geoscience Department of Geography & Geology Program Review 2007-2014 Self-Study December 2014 Self-Study Program Review Committee: Joanne Halls, Chair Michael Smith, Geoscience Graduate Coordinator Andrea Hawkes, Todd LaMaskin, Scott Nooner Executive Summary The UNC Wilmington Department of Geography and Geology began in 1970 and has grown to be a thriving and vital component of the Earth sciences at both undergraduate and graduate levels. The department currently has 19 tenure-track faculty, 2 lecturers, 1.5 staff people who support four undergraduate (geography, geoscience, geology, and oceanography) and two graduate (geoscience and geospatial technology) programs. Within the past 5 years the department has had several changes in faculty composition through retirements/departures and new hires. The degree programs have also changed substantially where the MS in Geology has become the MS in Geoscience. The result is a growth in our graduate program from 24 students in 2013 to 43 students in 2014. The greatest challenge that has consistently affected the Department is space. The faculty are spread across campuses (Center for Marine Science and main campus) and buildings, there is insufficient research space for faculty and students on the main campus, and the space is in need of refurbishment to accommodate the latest trends in science and technology. The greatest challenge for the MS in Geoscience program are the student stipends. The number of stipends and the funding amount is far less than comparable programs within North Carolina and in the mid-Atlantic region. It is a strong testament to the recruiting efforts of Department faculty that given this low funding the program has grown so quickly over the past few years. -

Archaeology and Sclerochronology of Marine Bivalves
View metadata,Downloaded citation and from similar orbit.dtu.dk papers on:at core.ac.uk Mar 29, 2019 brought to you by CORE provided by Online Research Database In Technology Archaeology and sclerochronology of marine bivalves Butler, Paul G.; Freitas, Pedro Seabra; Burchell, Meghan; Chauvaud, Laurent Published in: Goods and Services of Marine Bivalves Link to article, DOI: 10.1007/978-3-319-96776-9_21 Publication date: 2018 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Butler, P. G., Freitas, P. S., Burchell, M., & Chauvaud, L. (2018). Archaeology and sclerochronology of marine bivalves. In A. C. Smaal, J. G. Ferreira, J. Grant, J. K. Petersen, & Ø. Strand (Eds.), Goods and Services of Marine Bivalves (pp. 413-444). Springer. DOI: 10.1007/978-3-319-96776-9_21 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Archaeology and Sclerochronology of Marine Bivalves
Downloaded from orbit.dtu.dk on: Sep 27, 2021 Archaeology and sclerochronology of marine bivalves Butler, Paul G.; Freitas, Pedro Seabra; Burchell, Meghan; Chauvaud, Laurent Published in: Goods and Services of Marine Bivalves Link to article, DOI: 10.1007/978-3-319-96776-9_21 Publication date: 2018 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Butler, P. G., Freitas, P. S., Burchell, M., & Chauvaud, L. (2018). Archaeology and sclerochronology of marine bivalves. In A. C. Smaal, J. G. Ferreira, J. Grant, J. K. Petersen, & Ø. Strand (Eds.), Goods and Services of Marine Bivalves (pp. 413-444). Springer. https://doi.org/10.1007/978-3-319-96776-9_21 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Chapter 21 Archaeology and Sclerochronology of Marine Bivalves Paul G. Butler, Pedro S. Freitas, Meghan Burchell, and Laurent Chauvaud Abstract In a rapidly changing world, maintenance of the good health of the marine environment requires a detailed understanding of its mechanisms of change, and the ability to detect early signals of a shift away from the equilibrium state that we assume characterized it before there was any significant human impact. -

(Conrad,1837) En Condiciones De Laboratorio
Programa de Estudios de Posgrado Efecto de la temperatura y alimentación en la maduración sexual del mejillón Modiolus capax (Conrad,1837) en condiciones de laboratorio TESIS Que para obtener el grado de Maestro en Ciencias Uso, Manejo y Preservación de los Recursos Naturales (Orientación en Acuicultura) P r e s e n t a Jesús Antonio López Carvallo La Paz, Baja California Sur, Septiembre del 2015 Conformación de Comité Comité Tutorial Dr. José Manuel Mazón Suástegui Director de tesis Centro de Investigaciones Biológicas del Noroeste, S.C Dr. Pedro Enrique Saucedo Lastra Co-Tutor Centro de Investigaciones Biológicas del Noroeste, S.C Dr. Ángel Isidro Campa Córdova Co-Tutor Centro de Investigaciones Biológicas del Noroeste, S.C Comité Revisor de Tesis Dr. José Manuel Mazón Suástegui Dr. Pedro Enrique Saucedo Lastra Dr. Ángel Isidro Campa Córdova Jurado de Examen de Grado Dr. José Manuel Mazón Suástegui Dr. Pedro Enrique Saucedo Lastra Dr. Ángel Isidro Campa Córdova Suplente Dr. Dariel Tovar Ramírez Resumen Modiolus capax es un mejillón nativo de Bahía de La Paz, B.C.S, México, con una amplia distribución geográfica y potencial de cultivo en áreas no aptas para otras especies de moluscos bivlavos comerciales como Crassostrea gigas y Nodipecten subnodosus. La recolecta de semilla silvestre no es suficiente para sostener una producción acuícola a futuro, por lo que es indispensable producirla en laboratorio, y para ello se requiere conocer su biología reproductiva y su adaptabilidad al manejo zootécnico bajo condiciones controladas. Desafortunadamente, se sabe muy poco sobre el manejo de reproductores de M. capax en laboratorio. El presente estudio ha sido enfocado a generar nuevo conocimiento básico, aplicable al desarrollo de procedimientos y tecnología para el acondicionamiento gonádico y maduración sexual de la especie en ambiente controlado. -

THE FESTIVUS ISSN: 0738-9388 a Publication of the San Diego Shell Club
(?mo< . fn>% Vo I. 12 ' 2 ? ''f/ . ) QUfrl THE FESTIVUS ISSN: 0738-9388 A publication of the San Diego Shell Club Volume: XXII January 11, 1990 Number: 1 CLUB OFFICERS SCIENTIFIC REVIEW BOARD President Kim Hutsell R. Tucker Abbott Vice President David K. Mulliner American Malacologists Secretary (Corres. ) Richard Negus Eugene V. Coan Secretary (Record. Wayne Reed Research Associate Treasurer Margaret Mulliner California Academy of Sciences Anthony D’Attilio FESTIVUS STAFF 2415 29th Street Editor Carole M. Hertz San Diego California 92104 Photographer David K. Mulliner } Douglas J. Eernisse MEMBERSHIP AND SUBSCRIPTION University of Michigan Annual dues are payable to San Diego William K. Emerson Shell Club. Single member: $10.00; American Museum of Natural History Family membership: $12.00; Terrence M. Gosliner Overseas (surface mail): $12.00; California Academy of Sciences Overseas (air mail): $25.00. James H. McLean Address all correspondence to the Los Angeles County Museum San Diego Shell Club, Inc., c/o 3883 of Natural History Mt. Blackburn Ave., San Diego, CA 92111 Barry Roth Research Associate Single copies of this issue: $5.00. Santa Barbara Museum of Natural History Postage is additional. Emily H. Vokes Tulane University The Festivus is published monthly except December. The publication Meeting date: third Thursday, 7:30 PM, date appears on the masthead above. Room 104, Casa Del Prado, Balboa Park. PROGRAM TRAVELING THE EAST COAST OF AUSTRALIA Jules and Carole Hertz will present a slide program on their recent three week trip to Queensland and Sydney. They will also bring a display of shells they collected Slides of the Club Christmas party will also be shown. -
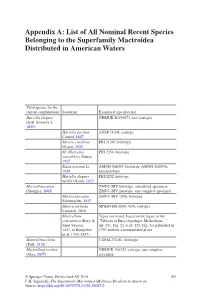
List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters
Appendix A: List of All Nominal Recent Species Belonging to the Superfamily Mactroidea Distributed in American Waters Valid species (in the current combination) Synonym Examined type material Harvella elegans NHMUK 20190673, two syntypes (G.B. Sowerby I, 1825) Harvella pacifica ANSP 51308, syntype Conrad, 1867 Mactra estrellana PRI 21265, holotype Olsson, 1922 M. (Harvella) PRI 2354, holotype sanctiblasii Maury, 1925 Raeta maxima Li, AMNH 268093, lectotype; AMNH 268093a, 1930 paralectotype Harvella elegans PRI 2252, holotype tucilla Olsson, 1932 Mactrellona alata ZMUC-BIV, holotype, articulated specimen; (Spengler, 1802) ZMUC-BIV, paratype, one complete specimen Mactra laevigata ZMUC-BIV 1036, holotype Schumacher, 1817 Mactra carinata MNHN-IM-2000-7038, syntypes Lamarck, 1818 Mactrellona Types not found, based on the figure of the concentrica (Bory de “Tableau of Encyclopedique Methodique…” Saint Vincent, (pl. 251, Fig. 2a, b, pl. 252, Fig. 2c) published in 1827, in Bruguière 1797 without a nomenclatorial act et al. 1791–1827) Mactrellona clisia USNM 271481, holotype (Dall, 1915) Mactrellona exoleta NHMUK 196327, syntype, one complete (Gray, 1837) specimen © Springer Nature Switzerland AG 2019 103 J. H. Signorelli, The Superfamily Mactroidea (Mollusca:Bivalvia) in American Waters, https://doi.org/10.1007/978-3-030-29097-9 104 Appendix A: List of All Nominal Recent Species Belonging to the Superfamily… Valid species (in the current combination) Synonym Examined type material Lutraria ventricosa MCZ 169451, holotype; MCZ 169452, paratype; -

Fujita Et Al
TRANS-HOLOCENE OCCUPATIONS AT CAÑADA DE LA ENFERMERÍA SURESTE #3 (SITE A-119), BAJA CALIFORNIA SUR, MEXICO HARUMI FUJITA, ANDREA HERNÁNDEZ, KARIM BULHUSEN INSTITUTO NACIONAL DE ANTROPOLOGÍA E HISTORIA (INAH), BAJA CALIFORNIA SUR, MEXICO AMIRA F. AINIS DEPARTMENT OF ANTHROPOLOGY, UNIVERSITY OF OREGON RENÉ L. VELLANOWETH DEPARTMENT OF ANTHROPOLOGY, CALIFORNIA STATE UNIVERSITY, LOS ANGELES. The eastern Cape Region contains ample evidence for human occupations spanning the past 12,000 years. Excavations at Site A-119, stretching north-south along a stream bank, add to our understanding of human lifeways throughout the Holocene. Radiocarbon dates extend from ~10,000 to 1800 cal BP, with vertical and horizontal stratigraphy suggesting multiple occupational episodes. Surface collections recorded an unusually high density of projectile points. Excavations uncovered an abundance of faunal remains and lithic artifacts, including side-notched projectile points, shell beads, shell fishhooks, and coral abraders. In spite of increasing urban development, it is imperative that we protect and record our cultural legacy on the Baja California peninsula. This paper focuses on recent excavations at A-119, a multi-component archaeological site located north-northeast of the city of La Paz. Several sites with components dated to the Terminal Pleistocene and Early Holocene have been located in the La Paz Bay area of the Cape Region, including on Espíritu Santo Island (Figure 1). Within the past several years we have conducted extensive surveys, recording numerous sites (Fujita 2010, 2014; Fujita et al. 2013; Fujita y Bulhusen 2014a y 2014b; Fujita and Poyatos 2007; Fujita and Hernández del Villar 2016; Fujita and Hernández Velázquez 2017; García et al. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste