Analysis of the Evolution of the Hominin Jaw & Dentition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
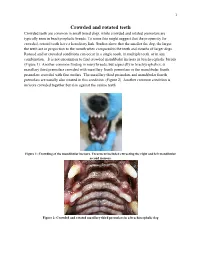
Crowded and Rotated Teeth Crowded Teeth Are Common in Small Breed Dogs, While Crowded and Rotated Premolars Are Typically Seen in Brachycephalic Breeds
1 Crowded and rotated teeth Crowded teeth are common in small breed dogs, while crowded and rotated premolars are typically seen in brachycephalic breeds. To some this might suggest that the propensity for crowded, rotated teeth have a hereditary link. Studies show that the smaller the dog, the larger the teeth are in proportion to the mouth when compared to the teeth and mouths of larger dogs. Rotated and/or crowded conditions can occur in a single tooth, in multiple teeth, or in any combination. It is not uncommon to find crowded mandibular incisors in brachycephalic breeds. (Figure 1). Another common finding in many breeds, but especially in brachycephalics, is maxillary third premolars crowded with maxillary fourth premolars or the mandibular fourth premolars crowded with first molars. The maxillary third premolars and mandibular fourth premolars are usually also rotated in this condition .(Figure 2) Another common condition is incisors crowded together but also against the canine teeth Figure 1: Crowding of the mandibular incisors. Treatment included extracting the right and left mandibular second incisors. Figure 2: Crowded and rotated maxillary third premolars in a brachiocephalic dog 2 Rotation and crowding can cause pain from chronic tooth on tooth contact. This might be compared to the pain that humans experience from a caries that has been overfilled. It is a condition that generally does not result in clinical signs; however, it can be quite painful. The chronic trauma resulting from tooth on tooth contact can lead to tooth non vitality. Rotation and crowding can also result in tooth on soft tissue contact, which can be not only painful but can result in soft tissue defects. -

Veterinary Dentistry Extraction
Veterinary Dentistry Extraction Introduction The extraction of teeth in the dog and cat require specific skills. In this chapter the basic removal technique for a single rooted incisor tooth is developed for multi-rooted and canine teeth. Deciduous teeth a nd feline teeth, particularly those affected by odontoclastic resorptive lesions, also require special attention. Good technique requires careful planning. Consider if extraction is necessary, and if so, how is it best accomplished. Review the root morphology and surrounding structures using pre-operative radiographs. Make sure you have all the equipment you need, and plan pre and post-operative management. By the end of this chapter you should be able to: ü Know the indications for extracting a tooth ü Unders tand the differing root morphology of dog and cat teeth ü Be able to select an extraction technique and equipment for any individual tooth ü Know of potential complications and how to deal with them ü Be able to apply appropriate analgesic and other treatment. Indications for Extraction Mobile Teeth Mobile teeth are caused by advanced periodontal disease and bone loss. Crowding of Teeth Retained deciduous canine. Teeth should be considered for extraction when they are interfering with occlusion or crowding others (e.g. supernumerary teeth). Retained Deciduous Teeth Never have two teeth of the same type in the same place at the same time. This is the rule of dental succession. Teeth in the Line of a Fracture Consider extracting any teeth in the line of a fracture of the mandible or maxilla. Teeth Destroyed by Disease Teeth ruined by advanced caries, feline neck lesions etc. -

Feline Dentistry: Cats Are Not Small Dogs Matt Lemmons, DVM, DAVDC Medvet Indianapolis Carmel, IN
Basics for Practitioners: Oral Anatomy and Pathology Matt Lemmons, DVM, DAVDC MedVet Indianapolis Carmel, IN Dentistry is truly a branch of medicine and surgery. A strong knowledge of normal anatomy and pathology is cornerstone to adequate diagnosis and treatment of diseases of the oral cavity. The majority of oral related disease is inflammatory (periodontal disease) or traumatic (fractured teeth, orthopedic injuries) in nature. However other causes are not rare and need to be recognized. The basic dental unit is the tooth and surrounding periodontium. The tooth consists of the crown and root. The crown is covered in enamel and the root by cementum. Deep to the crown and cementum is the dentin. Dentin is a porous hard tissue which continuously grows toward the center of the tooth as long as the tooth is vital. Deep to the dentin is the pulp which consists of nerves, blood vessels, connective tissue, fibroblasts and odontoblasts. The periodontium is composed of the cementum, periodontal ligament, alveolar bone, and gingiva. The periodontal ligament serves to anchor the cementum to the alveolar bone, act as a shock absorber and aid in sensation. The gingiva is attached to the bone (attached gingiva), tooth by connective tissue and the most apical extent is not attached and is known as the free gingiva. The potential space between the free gingiva and tooth and ending apically at the sulcular epithelium is the gingival sulcus. In health this should be less than 3mm in depth in dogs and 1mm in cats. When addressing the teeth and periodontium, directional nomenclature is not similar to directional nomenclature of the rest of the body. -
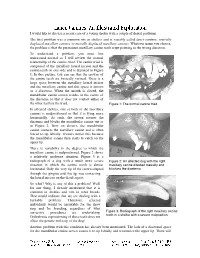
Lance Canines: an Illustrated Exploration I Would Like to Discuss a Recent Case of a Young Sheltie with a Couple of Dental Problems
Lance Canines: An Illustrated Exploration I would like to discuss a recent case of a young sheltie with a couple of dental problems. The first problem was a common one in shelties and is variably called lance canines, rostrally displaced maxillary canines or mesially displaced maxillary canines. Whatever name you choose, the problem is that the permanent maxillary canine teeth erupt pointing in the wrong direction. To understand a problem, you must first understand normal so I will review the normal relationship of the canine triad. The canine triad is composed of the maxillary lateral incisor and the canine teeth on one side and is depicted in Figure 1. In this picture, you can see that the crowns of the canine teeth are basically vertical. There is a large space between the maxillary lateral incisor and the maxillary canine and this space is known as a diastema. When the mouth is closed, the mandibular canine crown resides in the centre of the diastema so that it does not contact either of the other teeth in the triad. Figure 1: The normal canine triad. In affected shelties, one or both of the maxillary canines is malpositioned so that it is lying more horizontally. As such, the crown crosses the diastema and blocks the mandibular canine out as in Figure 2. Now on closure, the mandibular canine contacts the maxillary canine and is often forced to tip labially. Owners notice this because the mandibular canine then starts to catch on the upper lip. There is variability in the degree to which the maxillary canine is malpositioned. -

Chapter 15: Endodontics
Chapter 15 Endodontics. Chapter 15: Endodontics Endodontics is that branch of dentistry that deals outside dimension of the crown is established with the internal anatomy of the tooth and the early. Once the enamel is formed, the tissue that area where the inside of the tooth communicates made it goes dormant and no more enamel can with the rest of the body. ever be produced for that tooth. Teeth are composed of four main tissues. The Inside the tooth is the pulp. Lining the inside crown is covered by a thin veneer of enamel and wall of the developing tooth is a single layer of the root is covered by a thin layer of cementum. low columnar cells known as odontoblasts. Under the enamel and cementum is dentin and These cells produce the dentin. During pre- inside the dentin is a chamber filled with soft eruptive development and during eruption, the tissues known collectively as the dental pulp. odontoblasts produce primary dentin. Once the The chamber within the crown is called the pulp tooth has developed to its final length, the chamber and within the root it is called the root odontoblasts produce secondary dentin such that canal. the pulp chamber inside the tooth gets smaller as the wall of the tooth gets thicker. This The pulp is a highly organized collection of progression can be seen in the series of tissues that includes blood vessels, nerves, radiographs in Figure #15.1. Also review Figures lymphatic channels, undifferentiated cells and #7.4 to #7.8 on pages 27 to 29. -

Glossary of Commonly Used Dental Terms
Glossary of Commonly Used Dental Terms A • Abutment: A tooth or implant used to support a prosthesis. A crown unit used as part of a fixed bridge. • Abscess: A localized inflammation due to a collection of pus in the bone or soft tissue, usually caused by an infection. • Amalgam: A dental filling material, composed of mercury and other minerals, used to fill decayed teeth. • Alveoloplasty: A surgical procedure used to recontour the supporting bone struc tures in preparation of a complete or partial denture. • Anesthetic: A class of drugs that eliminated of reduces pain. See local anesthetic. • Anterior: Refers to the teeth and tissues located towards the front of the mouth (upper or lower incisors and canines). • Apex: The tip end of a root. • Apexification: A method of inducing apical closure, or the continual apical develop ment of the root of an incompletely formed tooth, in which the pulp is no longer vital. B • Bicuspid: A two-cuspid tooth found between the molar and the cuspid also known as an eye tooth or canine tooth. • Biopsy: A process of removing tissue to determine the existence of pathology. • Bitewing x-ray: X-rays taken of the crowns of teeth to check for decay. • Bleaching: The technique of applying a chemical agent, usually hydrogen peroxide, to the teeth to whiten them. • Bondin: A process to chemically etch the tooth's enamel to better attach ( bond ) composite filling material, veneers, or plastic/acrylic. • Bone loss: The breakdown and loss of the bone that supports the teeth, usually caused by infection or long-term occlusal ( chewing areas of the teeth ) stress. -

Dental Radiographs
The Importance of Dental Radiographs a b a a b b b b a Figure 7: Type I feline tooth resorption (TR). • Complete extraction of all roots is required for Type I TR. • These teeth have significant coronal resorption (a), but normal root structure (b). Figure 1: Normal dental radiograph, feline mandible. • Mandibular symphysis (a). • Canine roots (b). Roots of the canine teeth comprise the majority of the mandible. Figure 3: Advanced periodontal disease of the Figure 4: Small enamel fractures are very Figure 5: Seemingly normal teeth may be Figure 6: Feline tooth resorption (TR) is very mandible. common. infected. common. • Severe periodontal disease of the right lower quad- • Apparently healthy tooth with a small cusp fracture • This patient has one broken incisor (upper), but the • Minimal clinical evidence of pathology (upper) rant is evident on physical examination (upper). b d (upper). adjacent incisors appear normal. • Painful pathology missed without radiographs d • Radiograph reveals thin bone in the area of the first • This tooth is endodontically infected, as noted by • Radiograph reveals additional pathology (lower). (lower) molar (lower). the dark areas around the root tip (lower). Radiograph all teeth adjacent to pathologic teeth. Full mouth radiographs are recommended for all c Dental radiographs can help prevent iatrogenic • This infection would be missed without dental This illustrates the value of full-mouth dental a feline patients. damage (such as jaw fractures) during extractions. radiographs radiographs. c Figure 8: Type II feline tooth resorption (TR). Dental radiographs are required for all fractured teeth Often Type II TR results in replacement of tooth c to rule out hidden pathology. -

Maxillary Bilateral Canine-Premolar Transposition: a Rare Condition
Open Access Journal of Dentistry & Oral Disorders Case Report Maxillary Bilateral Canine-Premolar Transposition: A Rare Condition Agarwala P1, Agarwal V1, Rekhade R1 and Kulshrestha R2* Abstract 1Department of Orthodontics and Dentofacial Tooth transposition is very rare developmental phenomena in which Orthopaedics, Haldia Institute of Dental Sciences & the adjacent teeth are switched or have their positions changed with one Research, India another and due to this aesthetical and functional problem may be present. 2Consulting Orthodontist, Private Practice, Mumbai, The maxillary permanent canine is the tooth most frequently transposed, and India it is often transposed with the first premolar followed by the lateral incisor and *Corresponding author: Rohit Kulshrestha, lastly the central incisor in very rare cases. Several etiologic factors may lead Consulting Orthodontist, Private Practice, Mumbai, to transposition like genetics, developing tooth buds displacement, mechanical India interferences, trauma and early loss of incisors. This paper reports a case of bilateral transposition in the maxillary arch involving the first premolar and Received: March 28, 2017; Accepted: May 11, 2017; canine. Published: May 18, 2017 Keywords: Ectopic eruption; Maxillary canine; Tooth transposition Introduction interchanged positions between maxillary first premolar and canine on both right and the left sides (Figure 2). Upper left deciduous canine Tooth transposition is defined as the positional interchange in was still present and it showed decalcification on its labial aspect. spatial area of two adjacent teeth including their roots, or eruption Remaining teeth in all the other quadrants were at normal location and development of a tooth in a position normally occupied by a with normal morphology, overjet and overbite. -

Periodontal Disease in Cats
Periodontal Disease in Cats Periodontal disease is a progressive disease of the tissues surrounding the tooth root (see our handout on tooth anatomy for more information). As the tissues that support the teeth in its socket are destroyed, bacteria and by-products of the inflammation caused by the infection spread into the surrounding bone and can lead to sinus infections, bone loss and stress on the heart, liver and kidneys. Bone damage from infection can lead to pathologic fractures of the jaw (the jaw breaks during normal activity because it has been severely weakened by the chronic inflammation). In cats another manifestation of periodontal disease is called Buccal Bone Expansion. This looks as though the gum tissue and bone around the tooth are bulging out. They may also have extrusion, or hyper-eruption of their canine teeth that can make these teeth appear longer than they actually are. This cat has severe buccal bone expansion and extrusion affecting Another cat the canine tooth on demonstrating this side more than the the bone loss other. This is all due to associated with periodontal disease! BBE – one of the canine teeth even broke off due to the periodontal disease! Plaque is the cause of periodontal disease. It is an invisible film that forms on tooth surfaces containing saliva, food products and normal oral bacteria. Plaque can be removed with preventive care, so that the periodontal disease does not progress. Stage 1 Periodontal Disease is gingivitis. Gingivitis occurs when plaque remains on the tooth crown, causing inflammation of the gum line. As the first stage of periodontal disease, it is reversible with timely treatment. -

Williams and Corruccini 2007.1
29 The Relationship between Crown Size and Complexity in Two Collections Bridgett A. Williams and Robert S. Corruccini* Department of Anthropology, Southern Illinois University, Carbondale, IL ABSTRACT Many studies show human tooth crown size and in Newton Plantation slave remains from Barbados increases with increasing crown complexity (i.e., extra (736 dimensions of 368 teeth from ca. 100 individuals cusps, tubercles or grooves). Plio-Pleistocene hominid consisting of 8 post-canine types: mandibular premolars tooth size reduction has also incurred reduction in and molars, and maxillary molars). Significant positive complexity, which plays into many theories that attempt correlations show crown size and crown complexity to explain this well known, sustained odontometric decrease together, thus either type of data might serve to reduction. We correlated various types of tooth complexity document this decline. However the degree and pattern of with measured tooth size in two collections: the widely this positive correlation was distinct in the two samples. used ASU dental models (238 MD and BL dimensions Dental Anthropology 20:29-32. of 119 teeth involved in 19 post-incisor model plaques), Beginning in the Paleolithic and continuing through the positive selective pressures for small teeth. As tool use present, a Pleistocene through Holocene trend within the reduced the functions that were placed upon the jaw, human dentition has been recognized. Dental reduction, a large chewing muscles and a robust jaw forms were no reduction in tooth crown diameter, dramatically changed longer necessary. The teeth develop cryptically as a result the morphology of teeth. Though Europe demonstrated of genetic pressures, independent from jaw size selection. -

The Silent Pain of Pet Dental Disease
Copyright All contents copyright (c) 2011 by Sydney Pet Dentistry. All rights reserved. No part of this document or the related files may be reproduced or transmitted in any form, by any means (electronic, photocopying, recording, or otherwise) without the prior written permission of the publisher. Limit of Liability and Disclaimer of Warranty The publisher has used its best efforts in preparing this book, and the information provided herein is provided “as is.” Sydney Pet Dentistry makes no representation or warranties with respect to the accuracy or completeness of the contents of this book and specifically disclaims any implied warranties of merchantability or fitness for any particular purpose and shall in no event be liable for any loss of profit or any other commercial damage, including but not limited to special, incidental, consequential, or other damages. Trademarks This book identifies product names and services known to be trademarks, registered trademarks, or service marks of their respective holders. They are used throughout this book in an editorial fashion only. In addition, terms suspected of being trademarks, registered trademarks, or service marks have been appropriately capitalized, although Sydney Pet Dentistry cannot attest to the accuracy of this information. Use of a term in this book should not be regarded as affecting the validity of any trademark, registered trademark, or service mark. Sydney Pet Dentistry is not associated with any product or vendor mentioned in this book. Page II Table of Contents INTRODUctiON 1 Imagine having a toothache or sore mouth and not being able to tell anyone! 2 How to get the most out of this book 2 IS DENTAL DISEASE REALLY SUCH A BIG A DEAL? 3 SEVEN PET DENtiSTRY MYTHS - DEBUNKED! 9 MYTH #1: Bad breath is normal in pets. -

Dental Anatomy and Pathology
Dental anatomy and pathology Dental anatomy and pathology Geraldine Gorman Bay of Islands Veterinary Services, Shop 3, Selwyn Road, Paihia, Bay of Islands Introduction Various studies have shown that between 65 and 80% of dogs and cats over three years old have some form of oral disease and periodontal disease is the most common chronic infection seen in both humans and animals. Because Vet nurses are often the first point of contact between the client or pet and the practice it is essential that you have a basic knowledge of dental anatomy, pathology and procedures if you are to be a useful member of the dental team. In many cases a well-informed vet nurse can actually be a driver of dentistry in the workplace. Essential anatomy Dogs have 42 adult teeth, cats have 30. These are made up ofincisors, canines, premolars and molars. Incisors and canines are single while premolars and molars may be single, double or triple rooted. Figure 1. Canine Teeth. Diagram sourced from the Australia Dental Society Proceedings of the Annual Conference of the New Zealand Veterinary Nursing Association, 2012 6.03.1 Dental anatomy and pathology Figure 2. Feline teeth. Diagram sourced from the Australia Dental Society In cats there are less premolars with the firstmaxillary (upper) premolar and first and secondmandibular (lower) premolars missing. The incisors in both dogs and cats are numbered 1-3 from the centre towards the canine tooth. Navigating the Oral Cavity (Wiggs & Lobprise 1997) Labial surface: The surface of the incisors and canines directed toward the lips Buccal surface: The surface of the premolars and molars facing the cheek Facial surface: The labial and buccal surfaces collectively.