Coenonympha Tullia Nipisiquit)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A News and Events Diary from Wildlife and Conservation Groups in the Ipswich Area
Pantaloon Bee – see page 9 © Matt Garnham September - December 2018 A news and events Produced by the diary from wildlife and conservation groups in the Ipswich area BlueSnippets alien found White Admiral Lydia Woods in town Richard Stewart On the afternoon of Friday June 22nd my wife and I were walking down Westerfield Road in Ipswich and just past the gate into Christchurch Park we saw a white admiral on the pavement. It appeared to be a newly emerged While walking through Kiln Meadow on a warm adult but had probably been caught morning in July, I was more than a little surprised to in a vehicle slipstream. I cupped my hands around it, walked across the be confronted with a bright flash of blue! road and released the butterfly over the park railings. This was one of the On closer inspection I discovered a rather battered looking blue morpho butterfly new species I predicted for the park resting on the ground - not something you’d expect to see in Suffolk. These butterflies in future years as it has steadily been are generally found in Central and South America, although they are a popular choice colonising towards Ipswich. One was for butterfly houses – it’s likely this one escaped from the butterfly house situated at seen and photographed in The Dales Jimmy’s Farm. After taking some photos of this blue alien, I left the butterfly resting in a in 2015. With this in mind more patch of bindweed. While this was a lovely sight to see, hopefully it won’t be a regular honeysuckle, the larval food plant, occurrence. -
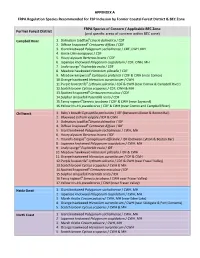
APPENDIX a FRPA Regulation Species Recommended for FSP
APPENDIX A FRPA Regulation Species Recommended for FSP Inclusion by Former Coastal Forest District & BEC Zone FRPA Species of Concern / Applicable BEC Zone Former Forest District (and specific areas of concern within BEC zone) B Campbell River 1. Dalmatian toadflax Linaria dalmatica / CDF B 2. Diffuse knapweed Centaurea diffusa / CDF 3. Giant knotweed Polygonum sachalinense / CDF, CWH, MH 4. Gorse Ulex europaeus / CDF 5. Hoary alyssum Berteroa incana / CDF 6. Japanese knotweed Polygonum cuspidatum / CDF, CWH, MH 7. Leafy spurgeB Euphorbia esula / CDF 8. Meadow hawkweed Hieracium pilosella / CDF 9. Meadow knapweedB Centaurea pratensis / CDF & CWH (near Comox) 10. Orange hawkweed Hieracium aurantiacum / CWH 11. Purple loosestrifeB Lythrum salicaria / CDF & CWH (near Comox & Campbell River) 12. Scotch broom Cytisus scoparius / CDF, CWH & MH 13. Spotted knapweedB Centaurea maculosa / CDF 14. Sulphur cinquefoil Potentilla recta / CDF 15. Tansy ragwortBSenecio jacobaea / CDF & CWH (near Sayward) 16.Yellow Iris Iris pseudacorus / CDF & CWH (near Comox and Campbell River) Chilliwack 1. Baby's breath Gypsophila paniculata / IDF (between Lillooet & Boston Bar) 2. Blueweed Echium vulgare / IDF & CWH 3. Dalmatian toadflaxBLinaria dalmatica / IDF 4. Diffuse knapweedB Centaurea diffusa / IDF 5. Giant knotweed Polygonum sachalinense / CWH, MH 6. Hoary alyssum Berteroa incana / IDF 7. Hound's-tongueB Cynoglossum officinale / IDF (between Lytton & Boston Bar) 8. Japanese knotweed Polygonum cuspidatum / CWH, MH 9. Leafy spurgeB Euphorbia esula / IDF 10. Meadow hawkweed Hieracium pilosella / IDF & CWH 11. Orange hawkweed Hieracium aurantiacum / IDF & CWH 12. Purple loosestrifeB Lythrum salicaria / CDF & CWH (near Fraser Valley) 13. Scotch broom Cytisus scoparius / CWH & MH 14. Spotted knapweedB Centaurea maculosa / IDF 15. Sulphur cinquefoil Potentilla recta / IDF 16. -

Superior National Forest
Admirals & Relatives Subfamily Limenitidinae Skippers Family Hesperiidae £ Viceroy Limenitis archippus Spread-wing Skippers Subfamily Pyrginae £ Silver-spotted Skipper Epargyreus clarus £ Dreamy Duskywing Erynnis icelus £ Juvenal’s Duskywing Erynnis juvenalis £ Northern Cloudywing Thorybes pylades Butterflies of the £ White Admiral Limenitis arthemis arthemis Superior Satyrs Subfamily Satyrinae National Forest £ Common Wood-nymph Cercyonis pegala £ Common Ringlet Coenonympha tullia £ Northern Pearly-eye Enodia anthedon Skipperlings Subfamily Heteropterinae £ Arctic Skipper Carterocephalus palaemon £ Mancinus Alpine Erebia disa mancinus R9SS £ Red-disked Alpine Erebia discoidalis R9SS £ Little Wood-satyr Megisto cymela Grass-Skippers Subfamily Hesperiinae £ Pepper & Salt Skipper Amblyscirtes hegon £ Macoun’s Arctic Oeneis macounii £ Common Roadside-Skipper Amblyscirtes vialis £ Jutta Arctic Oeneis jutta (R9SS) £ Least Skipper Ancyloxypha numitor Northern Crescent £ Eyed Brown Satyrodes eurydice £ Dun Skipper Euphyes vestris Phyciodes selenis £ Common Branded Skipper Hesperia comma £ Indian Skipper Hesperia sassacus Monarchs Subfamily Danainae £ Hobomok Skipper Poanes hobomok £ Monarch Danaus plexippus £ Long Dash Polites mystic £ Peck’s Skipper Polites peckius £ Tawny-edged Skipper Polites themistocles £ European Skipper Thymelicus lineola LINKS: http://www.naba.org/ The U.S. Department of Agriculture (USDA) prohibits discrimination http://www.butterfliesandmoths.org/ in all its programs and activities on the basis of race, color, national -

Some Butterfly Observations in the Karaganda Oblast of Kazakstan (Lepidoptera, Rhopalocera) by Bent Kjeldgaard Larsen Received 3.111.2003
©Ges. zur Förderung d. Erforschung von Insektenwanderungen e.V. München, download unter www.zobodat.at Atalanta (August 2003) 34(1/2): 153-165, colour plates Xl-XIVa, Wurzburg, ISSN 0171-0079 Some butterfly observations in the Karaganda Oblast of Kazakstan (Lepidoptera, Rhopalocera) by Bent Kjeldgaard Larsen received 3.111.2003 Abstract: Unlike the Ural Mountains, the Altai, and the Tien Shan, the steppe region of Cen tral Asia has been poorly investigated with respect to butterflies - distribution maps of the re gion's species (1994) show only a handful occurring within a 300 km radius of Karaganda in Central Kazakstan. It is therefore not surprising that approaching 100 additional species were discovered in the Karaganda Oblast during collecting in 1997, 2001 and 2002. During two days of collecting west of the Balkash Lake in May 1997, nine species were identified. On the steppes in the Kazakh Highland, 30 to 130 km south of Karaganda, about 50 butterflies were identified in 2001 and 2002, while in the Karkaralinsk forest, 200 km east of Karaganda, about 70 were encountered. Many of these insects are also to be found in western Europe and almost all of those noted at Karkaralinsk and on the steppes occur in South-Western Siberia. Observations revealed Zegris eupheme to be penetrating the area from the west and Chazara heydenreichi from the south. However, on the western side of Balkash Lake the picture ap peared to change. Many of the butterflies found here in 1997 - Parnassius apollonius, Zegris pyrothoe, Polyommatus miris, Plebeius christophi and Lyela myops - mainly came from the south, these belonging to the semi-desert and steppe fauna of Southern Kazakstan. -

Habitat Use and Population Structure of Protected Butterflies
DE TTK 1949 HABITAT USE AND POPULATION STRUCTURE OF PROTECTED BUTTERFLIES VÉDETT NAPPALI LEPKÉK ÉLŐHELYHASZNÁLATA ÉS POPULÁCIÓSZERKEZETE Egyetemi doktori (PhD) értekezés ÖRVÖSSY NOÉMI témavezető DR. VARGA ZOLTÁN DEBRECENI EGYETEM Természettudományi Doktori Tanács Juhász-Nagy Pál Doktori Iskola Debrecen, 2014. Ezen értekezést a Debreceni Egyetem Természettudományi Doktori Tanács Juhász-Nagy Pál Doktori Iskola Biodiverzitás programja keretében készítettem a Debreceni Egyetem természettudományi doktori (PhD) fokozatának elnyerése céljából. Debrecen, 2014. december 10. Örvössy Noémi Tanúsítom, hogy Örvössy Noémi doktorjelölt 2004- 2014 között a fent megnevezett Doktori Iskola Biodiverzitás programjának keretében irányításommal végezte munkáját. Az értekezésben foglalt eredményekhez a jelölt önálló alkotó tevékenységével meghatározóan hozzájárult. Az értekezés elfogadását javasolom. Debrecen, 2014. december 10. Prof. Dr. Varga Zoltán HABITAT USE AND POPULATION STRUCTURE OF PROTECTED BUTTERFLIES Értekezés a doktori (Ph.D.) fokozat megszerzése érdekében a biológia. tudományágban Írta: Örvössy Noémi okleveles biológus Készült a Debreceni Egyetem Juhász-Nagy Pál doktori iskolája (Biodiverzitás programja) keretében Témavezető: Dr. Varga Zoltán A doktori szigorlati bizottság: elnök: Dr. Pócsi István ....................................................... tagok: Dr. Rózsa Lajos ....................................................... Dr. Földvári Mihály ....................................................... A doktori szigorlat időpontja: 2013. február -

Newsletter No 250 July 2018
Published by RUGBY NATURAL HISTORY SOCIETY www.rugbynaturalhistory.org.uk PRESIDENT – Dr P Reeve Newsletter No 250 July 2018 Contents this edition ~Minibus trip: Rutland Water (book now!) ~News of members ~Summer field visit reports ~ Winter indoor meetings: dates for your diary ~Data protection information ~Current committee members (with contact information) Appendices included: species lists for Grove Hill, Snitterfield Bushes, Dunchurch Meadows, Stockton Cutting and Tasker’s Meadow Photos © Paul Hodges: cowslip carpet; thimble morel; semi-free morel at Grove Hill reserve Minibus trip? Speak up now! Rutland Water. Would you like to travel by minibus to our Rutland Water field visit on Thursday 6 September? Several members requested that we arrange this and David Knapp is willing to do so as long as there is sufficient interest - at least sixteen people would be 1 needed. The cost of a minibus would be £20 per person. The departure/return point would, as usual, be St Mark’s Church car park in Bilton, with additional pick up/drop off points in Long Itchington and Marton. The proposed return visit to Oxford Natural History Museum was cancelled because there were not enough people to make it viable. This is therefore now the FINAL CALL (!) for Rutland Water. If you would be interested in travelling by minibus, please let David know by Wednesday 1 August 2018 and he will then get back to you with further details. Tel. 01788 817346 or e:mail [email protected] News of members Most members will already know that Frank Ollerenshaw died in May. Nine of us attended his funeral, where we learned that he had served in young people’s organisations, as well as being a member both of the society and of Warwickshire Wildlife Trust and having many other interests. -

Land-Use Changes, Farm Management and the Decline of Butterflies Associated with Semi-Natural Grasslands in Southern Sweden
A peer-reviewed open-access journal Nature Conservation Land-use6: 31–48 (2013) changes, farm management and the decline of butterflies.... 31 doi: 10.3897/natureconservation.6.5205 APPLIED ECOLOGY http://www.pensoft.net/natureconservation Launched to accelerate biodiversity conservation Land-use changes, farm management and the decline of butterflies associated with semi-natural grasslands in southern Sweden Sven G. Nilsson1, Markus Franzén1,2, Lars B. Pettersson1,3 1 Biodiversity Unit, Department of Biology, Lund University, Ecology Building, SE-223 62 Lund, Sweden 2 UFZ Helmholtz Centre for Environmental Research, Department of Community Ecology, Theodor-Lieser- Straße 4, D-06120 Halle, Germany 3 Swedish Butterfly Monitoring Scheme, Lund University, Ecology Buil- ding, SE-223 62 Lund, Sweden Corresponding author: Lars B. Pettersson ([email protected]) Academic editor: L. Penev | Received 26 March 2013 | Accepted 30 October 2013 | Published 18 November 2013 Citation: Nilsson SG, Franzén M, Pettersson LB (2013) Land-use changes, farm management and the decline of butterflies associated with semi-natural grasslands in southern Sweden. Nature Conservation 18: 31–48. doi: 10.3897/ natureconservation.6.5205 Abstract Currently, we are experiencing biodiversity loss on different spatial scales. One of the best studied taxo- nomic groups in decline is the butterflies. Here, we review evidence for such declines using five systematic studies from southern Sweden that compare old butterfly surveys with the current situation. Additionally, we provide data on butterfly and burnet moth extinctions in the region’s counties. In some local areas, half of the butterfly fauna has been lost during the last 60–100 years. -

A Distinctive New Species of Cloud Forest Euptychiina (Lepidoptera: Nymphalidae: Satyrinae) from Ecuador and Peru
WILLMOTT ET AL.: New species of Erichthodes TROP. LEPID. RES., 28(1): 39-45, 2018 39 A distinctive new species of cloud forest Euptychiina (Lepidoptera: Nymphalidae: Satyrinae) from Ecuador and Peru Keith R. Willmott1, Gerardo Lamas2, James Radford3, Mario A. Marín4, Shinichi Nakahara1, Marianne Espeland5, Lei Xiao1, and Jason P. W. Hall6 1. McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL, USA: [email protected] 2. Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru. 3. Cambridge, UK. 4. Departamento de Biologia Animal and Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, Rua Monteiro Lobato, 255 - Cidade Universitária Zeferino Vaz - Barão Geraldo, 13083-862, Campinas, São Paulo, Brazil. 5. Arthropoda Department, Zoological Research Museum Alexander Koenig, Adenauer Allee 160, 53113 Bonn, Germany. 6. Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA Date of issue online: 13 July 2018 Zoobank Registered: urn:lsid:zoobank.org:pub:F4A0F8EB-600F-4973-9D52-DDA7E27C3EF8 Electronic copies (ISSN 2575-9256) in PDF format at: http://journals.fcla.edu/troplep; https://zenodo.org; archived by the Institutional Repository at the University of Florida (IR@UF), http://ufdc.ufl.edu/ufir;DOI : 10.5281/zenodo.1309677 © The author(s). This is an open access article distributed under the Creative Commons license CC BY-NC 4.0 (https://creativecommons.org/ licenses/by-nc/4.0/). Abstract: A new species of Euptychiina, Erichthodes eremita Lamas, Willmott & Radford, n. sp., is described and illustrated. DNA sequence data suggest that the new species is sister to a species currently placed in Erichthodes Forster, 1964, although ongoing revision of the generic taxonomy of the subtribe might result in the reclassification of both of these species in future. -

The Radiation of Satyrini Butterflies (Nymphalidae: Satyrinae): A
Zoological Journal of the Linnean Society, 2011, 161, 64–87. With 8 figures The radiation of Satyrini butterflies (Nymphalidae: Satyrinae): a challenge for phylogenetic methods CARLOS PEÑA1,2*, SÖREN NYLIN1 and NIKLAS WAHLBERG1,3 1Department of Zoology, Stockholm University, 106 91 Stockholm, Sweden 2Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Av. Arenales 1256, Apartado 14-0434, Lima-14, Peru 3Laboratory of Genetics, Department of Biology, University of Turku, 20014 Turku, Finland Received 24 February 2009; accepted for publication 1 September 2009 We have inferred the most comprehensive phylogenetic hypothesis to date of butterflies in the tribe Satyrini. In order to obtain a hypothesis of relationships, we used maximum parsimony and model-based methods with 4435 bp of DNA sequences from mitochondrial and nuclear genes for 179 taxa (130 genera and eight out-groups). We estimated dates of origin and diversification for major clades, and performed a biogeographic analysis using a dispersal–vicariance framework, in order to infer a scenario of the biogeographical history of the group. We found long-branch taxa that affected the accuracy of all three methods. Moreover, different methods produced incongruent phylogenies. We found that Satyrini appeared around 42 Mya in either the Neotropical or the Eastern Palaearctic, Oriental, and/or Indo-Australian regions, and underwent a quick radiation between 32 and 24 Mya, during which time most of its component subtribes originated. Several factors might have been important for the diversification of Satyrini: the ability to feed on grasses; early habitat shift into open, non-forest habitats; and geographic bridges, which permitted dispersal over marine barriers, enabling the geographic expansions of ancestors to new environ- ments that provided opportunities for geographic differentiation, and diversification. -

Oregon Silverspot Recovery Plan
PART I INTRODUCTION Overview The Oregon silverspot butterfly (Speyeria zerene hippolyta) is a small, darkly marked coastal subspecies of the Zerene fritillary, a widespread butterfly species in montane western North America. The historical range of the subspecies extends from Westport, Grays Harbor County, Washington, south to Del Norte County, California. Within its range, the butterfly is known to have been extirpated from at least 11 colonies (2 in Washington, 8 in Oregon, and 1 in California). We, the U.S. Department of the Interior, Fish and Wildlife Service, listed the Oregon silverspot butterfly was listed as a threatened species with critical habitat in 1980 (USDI 1980; 45 FR 44935). We completed a recovery plan for this species in 1982 (USDI 1982). The species recovery priority number is 3, indicating a high degree of threat and high recovery potential (USDI 1983; 48 FR 43098). At the time of listing, the only viable population known was at Rock Creek-Big Creek in Lane County, Oregon, and was managed by the U.S. Forest Service (Siuslaw National Forest). The Siuslaw National Forest developed an implementation plan (Clady and Parsons 1984) to guide management of the species at Rock Creek-Big Creek and Mount Hebo (Mt. Hebo) in Tillamook County, Oregon. Additional Oregon silverspot butterfly populations were discovered at Cascade Head, Bray Point, and Clatsop Plains in Oregon, on the Long Beach Peninsula in Washington, and in Del Norte County in California. The probability of survival of four populations has been increased by management efforts of the Siuslaw National Forest and The Nature Conservancy, however, some threats to the species remain at all of the sites. -

Invertebrates – a Forgotten Group of Animals In
INVERTEBRATES – A FORGOTTEN GROUP OF ANIMALS IN INFRASTRUCTURE PLANNING? BUTTERFLIES AS TOOLS AND MODEL ORGANISMS IN SWEDEN John Askling (Phone: +46 13 12 25 75, Email: [email protected]), Calluna AB, Linköpings slot, SE-582 28 Linköping, Sweden, Fax: +46 13 12 65 95, and Karl-Olof Bergman, (Phone: +46 13 28 26 85, Email: [email protected]), Department of Biology, Linköping University, SE-581 82 Linköping, Sweden, Fax: +46 13 28 13 99 Abstract: There is a growing concern about the ecological effects of roads and railways on animals. There is increased mortality due to road kills, changes in movement patterns and changes in the physical environment in areas affected by infrastructure. A majority of all studies have been on larger mammals. There are also a growing number of studies on smaller animals like birds, amphibians and small mammals. However, the studies of invertebrates are few in comparison with vertebrates, and the knowledge of the effects of infrastructure on this group is limited. The importance of also including invertebrates in the studies of infrastructure is evident. First of all, this group of animals is the richest of species that exists. They are also ecologically important. In Sweden, a majority of the red-listed species are invertebrates. Of 4,120 red-listed species, fully 2,337 are invertebrates. Their generation times are fast, which also makes the response on changes in their environment fast, compared to mammals and birds. For that reason, invertebrates can be expected to give an indication earlier than mammals if an area is negatively affected by infrastructure. -

Satyrijdae) ~~Edwards
STUDIES OF NEARCTJC (RHOPALOCERA, SATYRIJDAE) COENON0YMPHA TULLIA INORNATA 0 ~~EDWARDS F. MARTIN BROWN :ULEI .~~~~O THE -AMERICAN MUSEUM OF NATURAL HISTORY~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ rVLM .0: ARIL NvVYR:15 4 STUDIES OF NEARCTIC COENONYMPHA TULLIA (RHOPALOCERA, SATYRIDAE) STUDIES OF NEARCTIC COENONYMPHA TULLIA (RHOPALOCERA, SATYRIDAE) COENONYMPHA TULLIA INORNATA EDWARDS F. MARTIN BROWN Fountain Valley School Colorado Springs, Colorado BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY VOLUME 105 : ARTICLE 4 NEW YORK : 1955 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Volume 105, article 4, pages 359-410, text figures 1-19, plates 28, 29, tables 1-21 Issued March 14, 1955 Price: $.75 a copy INTRODUCTION THE INSECTS that compose the superspecies western parts of the Great Basin and the tullia are quite variable. Apparently they are southern part of Oregon is a small cluster of sensitive to environmental conditions and as forms that have these characters in common: a whole represent plastic and probably they are ocellated, very pale in color, and are "young" species. The shuttling about that clearly two brooded. This is california West- has been forced upon these insects in North wood and Hewitson. Two other names are America by the vacillation of the Pleistocene applied to these insects: galactinus and ice sheets has caused considerable differentia- eryngii. The former seems to be merely a tion among populations. When series from brood name; the latter may represent some well-separated localities are studied they ap- degree of hybridization with the adjacent pear to represent sharply defined species and form, ampelos. subspecies. That this is an illusion can be On the west coast, north of California, and demonstrated by study of material from in the basin-range region, there flies a group many localities across the continent.