Multisystem Trauma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Measuring Injury Severity
Measuring Injury Severity A brief introduction Thomas Songer, PhD University of Pittsburgh [email protected] Injury severity is an integral component in injury research and injury control. This lecture introduces the concept of injury severity and its use and importance in injury epidemiology. Upon completing the lecture, the reader should be able to: 1. Describe the importance of measuring injury severity for injury control 2. Describe the various measures of injury severity This lecture combines the work of several injury professionals. Much of the material arises from a seminar given by Ellen MacKenzie at the University of Pittsburgh, as well as reference works, such as that by O’Keefe. Further details are available at: “Measuring Injury Severity” by Ellen MacKenzie. Online at: http://www.circl.pitt.edu/home/Multimedia/Seminar2000/Mackenzie/Mackenzie.ht m O’Keefe G, Jurkovich GJ. Measurement of Injury Severity and Co-Morbidity. In Injury Control. Rivara FP, Cummings P, Koepsell TD, Grossman DC, Maier RV (eds). Cambridge University Press, 2001. 1 Degrees of Injury Severity Injury Deaths Hospitalization Emergency Dept. Physician Visit Households Material in the lectures before have spoken of the injury pyramid. It illustrates that injuries of differing levels of severity occur at different numerical frequencies. The most severe injuries occur less frequently. This point raises the issue of how do you compare injury circumstances in populations, particularly when levels of severity may differ between the populations. 2 Police EMS Self-Treat Emergency Dept. doctor Injury Hospital Morgue Trauma Center Rehab Center Robertson, 1992 For this issue, consider that injuries are often identified from several different sources. -

A Rare Case of Penetrating Trauma of Frontal Sinus with Anterior Table Fracture Himanshu Raval1*, Mona Bhatt2 and Nihar Gaur3
ISSN: 2643-4474 Raval et al. Neurosurg Cases Rev 2020, 3:046 DOI: 10.23937/2643-4474/1710046 Volume 3 | Issue 2 Neurosurgery - Cases and Reviews Open Access CASE REPORT Case Report: A Rare Case of Penetrating Trauma of Frontal Sinus with Anterior Table Fracture Himanshu Raval1*, Mona Bhatt2 and Nihar Gaur3 1 Department of Neurosurgery, NHL Municipal Medical College, SVP Hospital Campus, Gujarat, India Check for updates 2Medical Officer, CHC Dolasa, Gujarat, India 3GAIMS-GK General Hospital, Gujarat, India *Corresponding author: Dr. Himanshu Raval, Resident, Department of Neurosurgery, NHL Municipal Medical College, SVP Hospital Campus, Elisbridge, Ahmedabad, Gujarat, 380006, India, Tel: 942-955-3329 Abstract Introduction Background: Head injury is common component of any Road traffic accident (RTA) is the most common road traffic accident injury. Injury involving only frontal sinus cause of cranio-facial injury and involvement of frontal is uncommon and unique as its management algorithm is bone fractures are rare and constitute 5-9% of only fa- changing over time with development of radiological modal- ities as well as endoscopic intervention. Frontal sinus inju- cial trauma. The degree of association has been report- ries may range from isolated anterior table fractures causing ed to be 95% with fractures of the anterior table or wall a simple aesthetic deformity to complex fractures involving of the frontal sinuses, 60% with the orbital rims, and the frontal recess, orbits, skull base, and intracranial con- 60% with complex injuries of the naso-orbital-ethmoid tents. Only anterior table injury of frontal sinus is rare in pen- region, 33% with other orbital wall fractures and 27% etrating head injury without underlying brain injury with his- tory of unconsciousness and questionable convulsion which with Le Fort level fractures. -

Traumatic Brain Injury
REPORT TO CONGRESS Traumatic Brain Injury In the United States: Epidemiology and Rehabilitation Submitted by the Centers for Disease Control and Prevention National Center for Injury Prevention and Control Division of Unintentional Injury Prevention The Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation is a publication of the Centers for Disease Control and Prevention (CDC), in collaboration with the National Institutes of Health (NIH). Centers for Disease Control and Prevention National Center for Injury Prevention and Control Thomas R. Frieden, MD, MPH Director, Centers for Disease Control and Prevention Debra Houry, MD, MPH Director, National Center for Injury Prevention and Control Grant Baldwin, PhD, MPH Director, Division of Unintentional Injury Prevention The inclusion of individuals, programs, or organizations in this report does not constitute endorsement by the Federal government of the United States or the Department of Health and Human Services (DHHS). Suggested Citation: Centers for Disease Control and Prevention. (2015). Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. Atlanta, GA. Executive Summary . 1 Introduction. 2 Classification . 2 Public Health Impact . 2 TBI Health Effects . 3 Effectiveness of TBI Outcome Measures . 3 Contents Factors Influencing Outcomes . 4 Effectiveness of TBI Rehabilitation . 4 Cognitive Rehabilitation . 5 Physical Rehabilitation . 5 Recommendations . 6 Conclusion . 9 Background . 11 Introduction . 12 Purpose . 12 Method . 13 Section I: Epidemiology and Consequences of TBI in the United States . 15 Definition of TBI . 15 Characteristics of TBI . 16 Injury Severity Classification of TBI . 17 Health and Other Effects of TBI . -

Retrospective Review of Gunshot Injuries to the Foot & Ankle
Retrospective Review of Gunshot Injuries to the Foot & Ankle; A New Classification and Treatment Protocol Richard Bauer, DPM (PGY-4); Eliezer Eisenberger, DPM (PGY-4); Faculty: Emilio Goez, DPM, FACFAS St. Barnabas Health System; Regional Level-1 Trauma Center - Bronx, NY Statement of Purpose Literature Review Results Analysis & Discussion cont... The U.S. has an average of 30,900 gun related deaths per year and an additional Much of the literature related to gunshot wounds is adapted from high velocity projectile combat injuries, however most There were a total of twelve (12) patients who met our inclusion criteria that were treated for isolated gunshot wounds to the We have divided the anatomic locations into “zones.” Zone 1 refers to the digits in their entirety up to and including the 69,863 non-fatal gun related injuries were reported in 20081. Several studies have civilian gunshot wounds are resultant from low velocity (<300 m/sec) firearms1,3,4. Gunshot wounds to the lower extremity foot and ankle between the dates of 8/1/2010-11/1/2012. All patients were male with a mean age of 24.25 years. All injuries metatarsophalangeal joints (MPJ‟s), Zone 2 refers to the metatarsal and tarsal bones and Zone 3 refers to the calcaneus, reviewed treatment protocols for gunshot injuries to bone and related structures, represent approximately 63% of all gunshot related injuries, however only a fraction of these are located in the foot & ankle4. were classified as low velocity gunshot wounds. Four patients (33.3%) presented with isolated digital injury, three (25%) talus, tibia and fibula. -

Thoracic and Abdominal Trauma
INJURIESINJURIES TOTO THETHE TRUNKTRUNK THROACIC AND ABDOMINAL INJURIES INITIALINITIAL ASSESSMENTASSESSMENT 1. PRIMARY SURVEY (1. MIN) 2. VITAL FUNCTIONS TREAT LIFE THREATENING FIRST 3. SECONDARY SURVEY 4. DEFINITIVE CARE A.B.C.D.E. LIFELIFE THREATENINGTHREATENING INJURIESINJURIES A. INJURIES TO THE AIRWAYS B. TENSION PTX SUCKING CHEST WOUND MASSIVE HEMOTHORAX FLAIL CHEST C. CARDIAC TAMPONADE MASSIVE HEMOTHORAX LIFELIFE THREATENINGTHREATENING CHESTCHEST INJURIESINJURIES •PNEUMOTHORAX •HEMOTHORAX •PULMONARY CONTUSION •TRACHEBRONCHIAL TREE INJURY •BLUNT CARDIAC INJURY •TRAUMATIC AORTIC INJURY •TRAMATIC DIAPHRAGMATIG INJURY •MEDIASTINAL TRANSVERSING WOUNDS PNEUMOTHORAXPNEUMOTHORAX AIR BETWEEN THE PARIETAL AND VISCERAL PLEURA RIB FRACTURES INJURIES TO THE LUNG INJURIES TO THE AIRWAYS BULLAS IATROGENIG FROM THE RETROPERITONEUM PNEUMOTHORAXPNEUMOTHORAX 1. 2. TENSIONTENSION PNEUMOTHORAXPNEUMOTHORAX ONE WAY VALVE – AIR FROM THE LUNG OR THROUGH THE CHEST WALL INTO THE THORACIC CAVITY CONSEQUENCE: HYPOXIA, BLOCKING OF THE VENOUS INFLOW CHEST PAIN, AIR HUNGER, HYPOTENSION, NECK VEIN DISTENSION, TACHYCARDIA CARDIAC TAMPONADE – NO BREATH SOUNDS IMMEDIATE TREATMENT TENSIONTENSION PNEUMOTHORAXPNEUMOTHORAX TENSION PTX NEEDLE THORACOCENTESIS HEMOTHORAXHEMOTHORAX BLOOD IN THE THORACIC CAVITY LUNG LACERATION RIB FRACTURE INTERCOSTAL VESSEL INJURY ART. MAMMARY INJURY PENETRATING OR BLUNT INJURY HEMOTHORAXHEMOTHORAX 1. ? 2. ! HTXHTX HEMOTHORAXHEMOTHORAX TREATMENT : CHEST TUBE – THORACOTOMY IS RARELY INDICATED THORACOTOMY: 1500 ML / DRAINAGE OR 200 ML/ HOUR -

Degloving Injury: Different Ways of Management 76
CASE REPORT Journal of Nepalgunj Medical College, 2018 Degloving Injury: Different Ways of Management Nakarmi KK1, Shrestha SP2 ABSTRACT Degloving injury involves shearing of the skin from the underlying tissue due to differential gliding in response to the tangential force applied to the surface of the body leading to disruption of all the blood vessels connected to skin. The flap of degloved skin has precarious blood supply making it almost impossible for the flap to survive. We describe two cases of degloving of thigh managed differently in different settings. Keywords: degloving; excision; split skin graft INTRODUCTION management of these patients have been associated with Degloving injuries occur when there is sufficient tangential force lesser number of surgeries and shorter hospital stay6. Clinical to a body surface to disrupt the structures connecting skin and evaluation aided by use of fluorescein dye to assess the flap subcutaneous tissues to the superficial fascia. There may also be viability will guide whether skin can be harvested from the associated injuries to the underlying soft tissues, bone, nerves flap which can be stored for later use if general condition and vessels. It involves the young males, and most are related to does not allow immediate grafting7. Use of the degloved flap road traffic accident constituting upto 4% of all the trauma after defatting along with negative pressure wound therapy related admissions. Treatment guidelines are not clear1. The has also been described8. injury may be so severe that the limb is non-viable and requires amputation. It has been classified into three group based on Cases whether only skin, underlying soft tissue or bone is involved in Case 1 the injury process2. -

Anesthesia for Trauma
Anesthesia for Trauma Maribeth Massie, CRNA, MS Staff Nurse Anesthetist, The Johns Hopkins Hospital Assistant Professor/Assistant Program Director Columbia University School of Nursing Program in Nurse Anesthesia OVERVIEW • “It’s not the speed which kills, it’s the sudden stop” Epidemiology of Trauma • ~8% worldwide death rate • Leading cause of death in Americans from 1- 45 years of age • MVC’s leading cause of death • Blunt > penetrating • Often drug abusers, acutely intoxicated, HIV and Hepatitis carriers Epidemiology of Trauma • “Golden Hour” – First hour after injury – 50% of patients die within the first seconds to minutesÆ extent of injuries – 30% of patients die in next few hoursÆ major hemorrhage – Rest may die in weeks Æ sepsis, MOSF Pre-hospital Care • ABC’S – Initial assessment and BLS in trauma – GO TEAM: role of CRNA’s at Maryland Shock Trauma Center • Resuscitation • Reduction of fractures • Extrication of trapped victims • Amputation • Uncooperative patients Initial Management Plan • Airway maintenance with cervical spine protection • Breathing: ventilation and oxygenation • Circulation with hemorrhage control • Disability • Exposure Initial Assessment • Primary Survey: – AIRWAY • ALWAYS ASSUME A CERVICAL SPINE INJURY EXISTS UNTIL PROVEN OTHERWISE • Provide MANUAL IN-LINE NECK STABILIZATION • Jaw-thrust maneuver Initial Assessment • Airway cont’d: – Cervical spine evaluation • Cross table lateral and swimmer’s view Xray • Need to see all seven cervical vertebrae • Only negative CT scan R/O injury Initial Assessment • Cervical -

Surgical Case Conference
Surgical Case Conference HENRY R. GOVEKAR, M.D. PGY-2 UNIVERSITY OF COLORADO HOSPITAL Objectives Introduce Patient Hospital Course Management of Injuries Decisions to make Outcome History and Physical 50y/o M who was transferred to Denver Health on post injury day #3. Patient involved in motorcycle collision in Vail; admit to local hospital. Patient riding with female companion, tight turn, laid down the bike on road, companion landing on top of patient. Companion suffered minor injuries including abrasions and knee pain History and Physical Our patient – c/o Right shoulder pain, left flank pain PMH: GERD; peptic ulcer PSH: hx of surgery for intractable ulcerative disease – distal gastrectomy with Billroth I, ? Of revision to Roux-en-Y gastrojejunostomy Meds: prilosec Social: denies tobacco; quit ETOH 13 years ago after MVC; denies IVDA NKDA History and Physical Exam per OSH: a&ox3, minor distress HR 110’s, BP 130/70’s, on NRB mask with sats 98% Multiple abrasions on scalp, arms , knees c/o right arm pain, left sided flank pain No other obvious injuries After fluid resuscitation – HD stable Sent to CT for abd/pelvis Post 10th rib fx Grade 3 splenic laceration Spleen Injury Scale Grade I Injury Grade IV injury http://emedicine.medscape.com/article/373694-media Grade V injury http://emedicine.medscape.com/article/373694-media What should our plan be? Management of Splenic Injuries Stable vs. Unstable Unstable – OR Stable – abd CT scan CT scan Other significant injury – OR Documented splenic injury Grade I, -
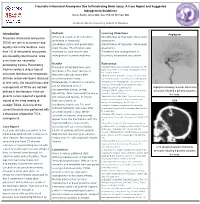
Traumatic Intracranial Aneurysms Due to Penetrating Brain Injury. a Case Report and Suggested Management Guidelines Breck Aaron Jones MD; Alex Patrick Michael MD
Traumatic Intracranial Aneurysms Due to Penetrating Brain Injury. A Case Report and Suggested Management Guidelines Breck Aaron Jones MD; Alex Patrick Michael MD Southern Illinois University School of Medicine Methods Learning Objectives Introduction Angiogram Traumatic intracranial aneurysms A Pubmed search of the literature Identification of traumatic intracranial pertaining to traumatic aneurysms. (TICA) are rare in occurrence and pseudoaneurysms and penetrating Classification of traumatic intracranial equally rare in the literature. Less brain trauma. The literature was aneurysms. than 1% of intracranial aneurysms reviewed for case reports and Treatment and management of are caused by blunt trauma, while management recommendations. traumatic intracranial aneurysms. even fewer are caused by penetrating trauma. Penetrating Results References Traumatic intracranial aneurysm 1.Aarabi B. Management of traumatic aneurysms caused by trauma creates a unique type of high-velocity missile head wounds. Neurosurg Clin N Am. formation is the most commonly aneurysm that does not incorporate Oct 1995;6(4):775-797. described vascular injury after 2.Rao GP, Rao NS, Reddy PK. Technique of removal of an all three vessel wall layers. Because penetrating brain injury. impacted sharp object in a penetrating head injury using the of their rarity, the natural history and Histologically, traumatic aneurysms lever principle. Br J Neurosurg. Dec 1998;12(6):569-571. 3.Vascular complications of penetrating brain injury. J can be described as true management of TICAs are not well Trauma. Aug 2001;51(2 Suppl):S26-28. Angiogram showing traumatic intracranial (incorporating intima, media, defined in the literature. Here we 4.Crompton MR. The pathogenesis of cerebral aneurysms. aneurysm following a gunshot wound to adventitia), false (incorporating one or Brain. -

Pre-Review Questionnaire Frequently Asked Questions
Pre-Review Questionnaire (PRQ) Frequently Asked Questions 1. Can abbreviations be used on the Pre-Review Questionnaire? Do not use abbreviations on the Pre-Review Questionnaire because abbreviations may not be the same from trauma care facility to trauma care facility. Write out all words and examples. 2. For the type of visit (question # 1 on the Pre-Review Questionnaire and Application Checklist form, DPH 7484), do I mark first visit or renewal visit? Mark the first visit this time. This is the first site visit made by the State of Wisconsin to your trauma care facility. The site visit means the actual physical site visit to your hospital. Filling out the assessment criteria document does not count as a site visit. The renewal box will be marked on your second site visit which will not start until at least 2010. This document is made so that the state does not have to create another Pre- Review Questionnaire document for future site visits. The renewal box can be checked and new information added or updated. 3. What are injury prevention initiatives and/or programs? Injuries cause death and disability. Strategies that decrease or prevent injury can improve the health of the community. Injury prevention initiatives and/or strategies focus primarily on environmental design, product design, human behavior, education, and legislative and regulatory requirements that support environmental and behavioral change (Center for Disease Control). Examples can include, but are not limited to: car seat clinics, bike helmet clinics, burn safety camp, farm safety, teen alcohol prevention programs (ENCARE, PARTY, Every 15 Minutes, etc…), ATV safety, hunting safety, snowmobiling safety, boating safety, poison control prevention, seat belt safety, etc… There are so many great national, state, and/or local programs to utilize or you can develop your own. -

Major Extremity Trauma Module
MAJOR EXTREMITY TRAUMA MODULE INTRODUCTION Extremity trauma in general is extremely common, and may be characterised by the following: • Occur in isolation, or in the multiply injured patient; • Be limb threatening and occasionally life threatening • Occur secondary to blunt or penetrating trauma • Present with degrees of severity from a closed, neurovascularly intact simple fracture through to a mangled extremity or traumatic amputation • Involve skeletal, soft tissue, vascular and neurological structures in various combinations While the principles of assessment are consistent irrespective of the severity of the injury, this module does not specifically address simple closed fractures. Rather, this module focuses on the assessment and management of more severe limb trauma and its complications. The most common mechanisms for major extremity trauma are open fractures, crush injuries and major soft tissue injury from motor vehicle crashes, pedestrian injuries, falls from heights and industrial accidents. 1 The lower limb is more frequently involved than the upper limb. Penetrating trauma resulting in vascular injuries is unfortunately increasing in frequency. Assessment and management of major extremity trauma must occur in the context of assessing and managing the patient as a whole. Life-threatening injuries, which should be identified as part of the primary survey, will always take precedence over limb-threatening injuries, which may not be identified until the secondary survey. Life threatening extremity injuries include: • Pelvic -

Treatment of Established Volkmann's Contracture*
~hop. Acta Treatment of Established Volkmann’sContracture* BY KENYA TSUGE, M.D.’J’, HIROSHIMA, JAPAN ldon, From the Department of Orthopaedic Surgery, Hiroshima Universi~.’ 1-76, School of Medicine, Hiroshima 38. The disease first described by Volkmann in 1881 is the extent of the disease: mild, moderate, and severe. In generally considered to result from spasm of the main ar- the mild type, also called the localized type, there was de- ~ts of teries of the forearm, and their branches as a consequence generation of part of the flexor digitorum profundus mus- Acta of trauma to the elbow or forearm. The severe and pro- cle, causing contractures in only two or three fingers. longed but incomplete interruption of arterial blood sup- There were hardly any neurological signs, and when pres- ~. (in ply, together with venostasis, produces acute ischemic ent they were minimum. In the moderate type, the muscle z and necrosis of the flexor muscles. The most marked ischemia degeneration involved all or nearly all of the flexor digito- occurs in the deeply situated muscles such as the flexor rum profundus and flexor pollicis longus, with partial pollicis longus and flexor digitorum profundus, but severe degeneration of the superficial muscles as well. The neu- ischemia is evident in the pronator teres and flexor rological signs were invariably present and generally -484, digitorum superficialis muscles, and comparatively mild the median nerve was more severely affected than the :rtag, ischemia occurs in the superficially located muscles such ulnar nerve. In the severe type, there was degeneration as the wrist flexors. The muscle degeneration which fol- of all the flexor muscles with necrosis in the center ).