Flavonoid Components of Different Color Magnolia Flowers and Their
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neighborwoods Right Plant, Right Place Plant Selection Guide
“Right Plant, Right Place” Plant Selection Guide Compiled by Samuel Kelleher, ASLA April 2014 - Shrubs - Sweet Shrub - Calycanthus floridus Description: Deciduous shrub; Native; leaves opposite, simple, smooth margined, oblong; flowers axillary, with many brown-maroon, strap-like petals, aromatic; brown seeds enclosed in an elongated, fibrous sac. Sometimes called “Sweet Bubba” or “Sweet Bubby”. Height: 6-9 ft. Width: 6-12 ft. Exposure: Sun to partial shade; range of soil types Sasanqua Camellia - Camellia sasanqua Comment: Evergreen. Drought tolerant Height: 6-10 ft. Width: 5-7 ft. Flower: 2-3 in. single or double white, pink or red flowers in fall Site: Sun to partial shade; prefers acidic, moist, well-drained soil high in organic matter Yaupon Holly - Ilex vomitoria Description: Evergreen shrub or small tree; Native; leaves alternate, simple, elliptical, shallowly toothed; flowers axillary, small, white; fruit a red or rarely yellow berry Height: 15-20 ft. (if allowed to grow without heavy pruning) Width: 10-20 ft. Site: Sun to partial shade; tolerates a range of soil types (dry, moist) Loropetalum ‘ZhuZhou’-Loropetalum chinense ‘ZhuZhou’ Description: Evergreen; It has a loose, slightly open habit and a roughly rounded to vase- shaped form with a medium-fine texture. Height: 10-15 ft. Width: 10-15ft. Site: Preferred growing conditions include sun to partial shade (especially afternoon shade) and moist, well-drained, acidic soil with plenty of organic matter Japanese Ternstroemia - Ternstroemia gymnanthera Comment: Evergreen; Salt spray tolerant; often sold as Cleyera japonica; can be severely pruned. Form is upright oval to rounded; densely branched. Height: 8-10 ft. Width: 5-6 ft. -

Indiana's Native Magnolias
FNR-238 Purdue University Forestry and Natural Resources Know your Trees Series Indiana’s Native Magnolias Sally S. Weeks, Dendrologist Department of Forestry and Natural Resources Purdue University, West Lafayette, IN 47907 This publication is available in color at http://www.ces.purdue.edu/extmedia/fnr.htm Introduction When most Midwesterners think of a magnolia, images of the grand, evergreen southern magnolia (Magnolia grandiflora) (Figure 1) usually come to mind. Even those familiar with magnolias tend to think of them as occurring only in the South, where a more moderate climate prevails. Seven species do indeed thrive, especially in the southern Appalachian Mountains. But how many Hoosiers know that there are two native species Figure 2. Cucumber magnolia when planted will grow well throughout Indiana. In Charles Deam’s Trees of Indiana, the author reports “it doubtless occurred in all or nearly all of the counties in southern Indiana south of a line drawn from Franklin to Knox counties.” It was mainly found as a scattered, woodland tree and considered very local. Today, it is known to occur in only three small native populations and is listed as State Endangered Figure 1. Southern magnolia by the Division of Nature Preserves within Indiana’s Department of Natural Resources. found in Indiana? Very few, I suspect. No native As the common name suggests, the immature magnolias occur further west than eastern Texas, fruits are green and resemble a cucumber so we “easterners” are uniquely blessed with the (Figure 3). Pioneers added the seeds to whisky presence of these beautiful flowering trees. to make bitters, a supposed remedy for many Indiana’s most “abundant” species, cucumber ailments. -

Thermogenesis, Flowering and the Association with Variation in Floral Odour Attractants in Magnolia Sprengeri (Magnoliaceae)
Thermogenesis, Flowering and the Association with Variation in Floral Odour Attractants in Magnolia sprengeri (Magnoliaceae) Ruohan Wang1, Sai Xu1,3, Xiangyu Liu2, Yiyuan Zhang1, Jianzhong Wang1, Zhixiang Zhang2* 1 National Engineering Laboratory for Tree Breeding, Key Laboratory for Genetics and Breeding of Forest Trees and Ornamental Plants, Ministry of Education, College of Biological Sciences and Biotechnology, Beijing Forestry University,Beijing, China, 2 Lab of Systematic Evolution and Biogeography of Woody Plants, College of Nature Conservation, Beijing Forestry University,Beijing, China, 3 School of Environment, Tsinghua University, Beijing, China Abstract Magnolia sprengeri Pamp. is an ornamentally and ecologically important tree that blooms at cold temperatures in early spring. In this study, thermogenesis and variation in the chemical compounds of floral odours and insect visitation in relation to flowering cycles were studied to increase our understanding of the role of floral thermogenesis in the pollination biology of M. sprengeri. There were five distinct floral stages across the floral cycle of this species: pre-pistillate, pistillate, pre- staminate, staminate and post-staminate. Floral thermogenesis during anthesis and consisted of two distinct peaks: one at the pistillate stage and the other at the staminate stage. Insects of five families visited M. sprengeri during the floral cycle, and sap beetles (Epuraea sp., Nitidulidae) were determined to be the most effective pollinators, whereas bees (Apis cerana, Apidae) were considered to be occasional pollinators. A strong fragrance was released during thermogenesis, consisting of 18 chemical compounds. Although the relative proportions of these compounds varied at different floral stages across anthesis, linalool, 1-iodo-2-methylundecane and 2,2,6-trimethyl-6-vinyltetrahydro-2H-pyran-3-ol were dominant. -

THE Magnoliaceae Liriodendron L. Magnolia L
THE Magnoliaceae Liriodendron L. Magnolia L. VEGETATIVE KEY TO SPECIES IN CULTIVATION Jan De Langhe (1 October 2014 - 28 May 2015) Vegetative identification key. Introduction: This key is based on vegetative characteristics, and therefore also of use when flowers and fruits are absent. - Use a 10× hand lens to evaluate stipular scars, buds and pubescence in general. - Look at the entire plant. Young specimens, shade, and strong shoots give an atypical view. - Beware of hybridisation, especially with plants raised from seed other than wild origin. Taxa treated in this key: see page 10. Questionable/frequently misapplied names: see page 10. Names referred to synonymy: see page 11. References: - JDL herbarium - living specimens, in various arboreta, botanic gardens and collections - literature: De Meyere, D. - (2001) - Enkele notities omtrent Liriodendron tulipifera, L. chinense en hun hybriden in BDB, p.23-40. Hunt, D. - (1998) - Magnolias and their allies, 304p. Bean, W.J. - (1981) - Magnolia in Trees and Shrubs hardy in the British Isles VOL.2, p.641-675. - or online edition Clarke, D.L. - (1988) - Magnolia in Trees and Shrubs hardy in the British Isles supplement, p.318-332. Grimshaw, J. & Bayton, R. - (2009) - Magnolia in New Trees, p.473-506. RHS - (2014) - Magnolia in The Hillier Manual of Trees & Shrubs, p.206-215. Liu, Y.-H., Zeng, Q.-W., Zhou, R.-Z. & Xing, F.-W. - (2004) - Magnolias of China, 391p. Krüssmann, G. - (1977) - Magnolia in Handbuch der Laubgehölze, VOL.3, p.275-288. Meyer, F.G. - (1977) - Magnoliaceae in Flora of North America, VOL.3: online edition Rehder, A. - (1940) - Magnoliaceae in Manual of cultivated trees and shrubs hardy in North America, p.246-253. -

STEVEN and ERIN FORD GARDEN Botanical Name Common Name
STEVEN AND ERIN FORD GARDEN Botanical Name Common Name Abutilon Flowering Maple Acer buergerianum Trident Maple Acer japonicum Full Moon Maple Acer palmatum Japanese Maple Acer palmatum 'Bloodgood' Japanese Maple Achillea millefolium Yarrow Adiantum Maidenhair Fern Agapanthus Lily of the Nile Ajuga Alcea rosea Ajuga Alcea rosea Hollyhock Anemone japonica Japanese Anemone Antirrhinum majus Snapdragon Aquilegia Columbine Aralia Japanese Aralia Artemisia stelleriana Dusty Miller Artemisia stelleriana Powis Castle Artemisia Asparagus densiflorus Asparagus Fern Asparagus densiflorus 'Sprengeri' Sprenger Asparagus Fern Asparagus retrofractus Ming Fern Azalea Azalea Azalea 'Hino' Basil Begonia Berberis thumbergii 'Golden Nugget' Japanese Barberry Bletilla striata Chinese Ground Orchid Boysenberries Buddleja Butterfly Bush Buxus sempervirens Common Boxwood Calendula officinalis Pot Marigold Camellia japonica Camellia sasanqua Carex oshimensis Sedge Carex variegata Sedge Caryopteris Bluebird Ceanothus Cedrus deodara Deodar Cedar Centranthus ruber Jupiter's Beard Cercis canadensis Eastern Rudbud Chives Chrysanthemum Chrysanthemum maximum Shasta Daisy Citrus Dwarf Blood Orange Citrus Dwarf Grapefruit 1 Citrus Dwarf Tangerine Citrus Navel Orange Citrus Variegated Lemon Clematis Cornus Dogwood Cornus kousa Kousa Dogwood Cornus stolonifera Redtwig Dogwood Cotinus Smoke Tree Cryptomeria japonica Japanese Cryptomeria Cyathea cooperi Australian Tree Fern Cyclamen Delphinium Dianella tasmanica 'Yellow Stripe' Flax Lily Dianthus Pink Dianthus barbatus Sweet -
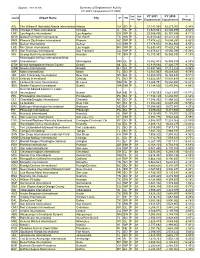
CY 2001 Compared to CY 2000
Source: 2001 ACAIS Summary of Enplanement Activity CY 2001 Compared to CY 2000 Svc Hub CY 2001 CY 2000 % Locid Airport Name City ST RO Lvl Type Enplanement Enplanement Change ATL The William B Hartsfield Atlanta International Atlanta GA SO P L 37,181,068 39,277,901 -5.34% ORD Chicago O'Hare International Chicago IL GL P L 31,529,561 33,845,895 -6.84% LAX Los Angeles International Los Angeles CA WP P L 29,365,436 32,167,896 -8.71% DFW Dallas/Fort Worth International Fort Worth TX SW P L 25,610,562 28,274,512 -9.42% PHX Phoenix Sky Harbor International Phoenix AZ WP P L 17,478,622 18,094,251 -3.40% DEN Denver International Denver CO NM P L 17,178,872 18,382,940 -6.55% LAS Mc Carran International Las Vegas NV WP P L 16,633,435 17,425,214 -4.54% SFO San Francisco International San Francisco CA WP P L 16,475,611 19,556,795 -15.76% IAH George Bush Intercontinental Houston TX SW P L 16,173,551 16,358,035 -1.13% Minneapolis-St Paul International/Wold- MSP Chamberlain/ Minneapolis MN GL P L 15,852,433 16,959,014 -6.53% DTW Detroit Metropolitan Wayne County Detroit MI GL P L 15,819,584 17,326,775 -8.70% EWR Newark International Newark NJ EA P L 15,497,560 17,212,226 -9.96% MIA Miami International Miami FL SO P L 14,941,663 16,489,341 -9.39% JFK John F Kennedy International New York NY EA P L 14,553,815 16,155,437 -9.91% MCO Orlando International Orlando FL SO P L 13,622,397 14,831,648 -8.15% STL Lambert-St Louis International St. -

American Forests National Big Tree Program Species Without Champions
American Forests National Big Tree Program Champion trees are the superstars of their species — and there are more than 700 of them in our national register. Each champion is the result of a lucky combination: growing in a spot protected by the landscape or by people who have cared about and for it, good soil, the right amount of water, and resilience to the elements, surviving storms, disease and pests. American Forests National Big Tree Program was founded to honor these trees. Since 1940, we have kept the only national register of champion trees (http://www.americanforests.org/explore- forests/americas-biggest-trees/champion-trees-national-register/) Champion trees are found by people just like you — school teachers, kids fascinated by science, tree lovers of all ages and even arborists for whom a fun day off is measuring the biggest tree they can find. You, too, can become a big tree hunter and compete to find new champions. Species without Champions (March, 2018) Gold rows indict species that have Idaho State Champions but the nominations are too old to be submitted for National Champion status. Scientific Name Species Common Name Abies lasiocarpa FIR Subalpine Acacia macracantha ACACIA Long-spine Acacia roemeriana CATCLAW Roemer Acer grandidentatum MAPLE Canyon or bigtooth maple Acer nigrum MAPLE Black Acer platanoides MAPLE Norway Acer saccharinum MAPLE Silver Aesculus pavia BUCKEYE Red Aesculus sylvatica BUCKEYE Painted Ailanthus altissima AILANTHUS Tree-of-heaven Albizia julibrissin SILKTREE Mimosa Albizia lebbek LEBBEK Lebbek -

Magnolia 'Galaxy'
Magnolia ‘Galaxy’ The National Arboretum presents Magnolia ‘Galaxy’, unique in form and flower among cultivated magnolias. ‘Galaxy’ is a single-stemmed, tree-form magnolia with ascending branches, the perfect shape for narrow planting sites. In spring, dark red-purple flowers appear after danger of frost, providing a pleasing and long-lasting display. Choose ‘Galaxy’ to shape up your landscape! Winner of a Pennsylvania Horticultural Society Gold Medal Plant Award, 1992. U.S. National Arboretum Plant Introduction Floral and Nursery Plants Research Unit U.S. National Arboretum, U.S. Department of Agriculture, Agricultural Research Service, 3501 New York Ave. NE., Washington, DC 20002 ‘Galaxy’ hybrid magnolia Botanical name: Magnolia ‘Galaxy’ (M. liliiflora ‘Nigra’ × M. sprengeri ‘Diva’) (NA 28352.14, PI 433306) Family: Magnoliaceae Hardiness: USDA Zones 5–9 Development: ‘Galaxy’ is an F1 hybrid selection resulting from a 1963 cross between Magnolia liliiflora ‘Nigra’ and M. sprengeri ‘Diva’. ‘Galaxy’ first flowered at 9 years of age from seed. The cultivar name ‘Galaxy’ is registered with the American Magnolia Society. Released 1980. Significance: Magnolia ‘Galaxy’ is unique in form and flower among cultivated magnolias. It is a single stemmed, pyramidal, tree-form magnolia with excellent, ascending branching habit. ‘Galaxy’ flowers 2 weeks after its early parent M. ‘Diva’, late enough to avoid most late spring frost damage. Adaptable to a wide range of soil conditions. Description: Height and Width: 30-40 feet tall and 22-25 feet wide. It reaches 24 feet in height with a 7-inch trunk diameter at 14 years. Moderate growth rate. Habit: Single-trunked, upright tree with narrow crown and ascending branches. -

Magnolia X Soulangiana Saucer Magnolia1 Edward F
Fact Sheet ST-386 October 1994 Magnolia x soulangiana Saucer Magnolia1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION Saucer Magnolia is a multi-stemmed, spreading tree, 25 feet tall with a 20 to 30-foot spread and bright, attractive gray bark (Fig. 1). Growth rate is moderately fast but slows down considerably as the tree reaches about 20-years of age. Young trees are distinctly upright, becoming more oval, then round by 10 years of age. Large, fuzzy, green flower buds are carried through the winter at the tips of brittle branches. The blooms open in late winter to early spring before the leaves, producing large, white flowers shaded in pink, creating a spectacular flower display. However, a late frost can often ruin the flowers in all areas where it is grown. This can be incredibly disappointing since you wait 51 weeks for the flowers to appear. In warmer climates, the late- flowering selections avoid frost damage but some are less showy than the early-flowered forms which blossom when little else is in flower. GENERAL INFORMATION Scientific name: Magnolia x soulangiana Figure 1. Middle-aged Saucer Magnolia. Pronunciation: mag-NO-lee-uh x soo-lan-jee-AY-nuh Common name(s): Saucer Magnolia DESCRIPTION Family: Magnoliaceae USDA hardiness zones: 5 through 9A (Fig. 2) Height: 20 to 25 feet Origin: not native to North America Spread: 20 to 30 feet Uses: container or above-ground planter; espalier; Crown uniformity: irregular outline or silhouette near a deck or patio; shade tree; specimen; no proven Crown shape: round; upright urban tolerance Crown density: open Availability: generally available in many areas within Growth rate: medium its hardiness range 1. -

Magnolia Obovata
ISSUE 80 INAGNOLN INagnolla obovata Eric Hsu, Putnam Fellow, Arnold Arboretum of Harvard University Photographs by Philippe de 8 poelberch I first encountered Magnolia obovata in Bower at Sir Harold Hillier Gardens and Arboretum, Hampshire, England, where the tightly pursed, waxy, globular buds teased, but rewarded my patience. As each bud unfurled successively, it emitted an intoxicating ambrosial bouquet of melons, bananas, and grapes. Although the leaves were nowhere as luxuriously lustrous as M. grandrflora, they formed an el- egant wreath for the creamy white flower. I gingerly plucked one flower for doser observation, and placed one in my room. When I re- tumed from work later in the afternoon, the mom was overpowering- ly redolent of the magnolia's scent. The same olfactory pleasure was later experienced vicariously through the large Magnolia x wiesneri in the private garden of Nicholas Nickou in southern Connecticut. Several years earlier, I had traveled to Hokkaido Japan, after my high school graduation. Although Hokkaido experiences more severe win- ters than those in the southern parts of Japan, the forests there yield a remarkable diversity of fora, some of which are popular ornamen- tals. When one drives through the region, the silvery to blue-green leaf undersides of Magnolia obovata, shimmering in the breeze, seem to flag the eyes. In "Forest Flora of Japan" (sggII), Charles Sargent commended this species, which he encountered growing tluough the mountainous forests of Hokkaido. He called it "one of the largest and most beautiful of the deciduous-leaved species in size and [the seed conesj are sometimes eight inches long, and brilliant scarlet in color, stand out on branches, it is the most striking feature of the forests. -

The Classics Shapes Magnolia & Imperial
THE CLASSICS SHAPES MAGNOLIA & IMPERIAL 1001 - WHITE + Gold Line 1002 - MAGNOLIA CREAM + Gold Line 1541 - JULIA GREEN + Gold Line MAGNOLIA (1021, 1032), PERSIA (1101, 1301) VOILE (1401), JULIA (1521, 1532, 1541) IRIS (1711), WHITE WITH GOLD LINE (1001) MAGNOLIA Shape MAGNOLIA (Shape Nr. 05) MAGNOLIA CREAM with Gold Line MAGNOLIA GREY with Platinum Line (Decor Nr. 1021) (Decor Nr. 1032) PERSIA Shape MAGNOLIA (Shape Nr. 05) PERSIA with Gold Line PERSIA CLASSIC with Gold Line (Decor Nr. 1101) (Decor Nr. 1301) VOILE VOILE with Gold Line (Decor Nr. 1401) WHITE Items of Shape MAGNOLIA (Shape Nr. 05) are also available in WHITE (Decor Nr. 1000) or WHITE with Gold Line (Decor Nr. 1001). See photo on cover page. JULIA Shape MAGNOLIA (Shape Nr. 05) JULIA GREY with Platinum Line (Decor Nr. 1532) JULIA BEIGE with Gold Line JULIA GREEN with Gold Line (Decor Nr. 1521) (Decor Nr. 1541) Shape IMPERIAL (Shape Nr. 04) JULIA BEIGE with Gold Line JULIA GREEN with Gold Line (Decor Nr. 1521) (Decor Nr. 1541) JULIA GREY with Platinum Line (Decor Nr. 1532) IRIS Shape MAGNOLIA (Shape Nr. 05) Shape IMPERIAL (Shape Nr. 04) IRIS PINK with Gold Line (Decor Nr. 1711) Shape IMPERIAL (Shape Nr. 04) Shape MAGNOLIA (Shape Nr. 05) Sugar 400 ml 04-....-6640-. Cup 250 ml Cup 370 ml Tea pot Cup 150 ml Cup 220 ml Cup 250 ml Cup 370 ml saucer 15 cm saucer 17 cm 1,5 L Creamer 250 ml saucer 13 cm saucer 14,5 cm saucer 15 cm saucer 17 cm 04-....-5125-. 04-....-5137-. -

Magnolia Quick Reference Guide
MagnoliaDr. Merrill x loebneri Magnolia 'Merrill' Hans Nelson Height: 30’ & Sons Nursery Width: 30’ Zone: 5 Foliage: Medium green leaves are slightly leath- ery. Bloom: Beautiful white, fragrant, star-shaped Magnolia Quick flowers. Bloom Size: 3” Bloom Time: March to April. Reference Guide Bark: Rough, light brown bark with streaks of light grey. Growth Habit: Upright habit, grown as a tree with low branches. MagnoliaAnn Magnolia liliflora 'Ann' Height: 10’ MagnoliaElizabeth acuminata Magnolia x denudata 'Elizabeth' Width: 10’ Height: 35’ Zone: 4 Width: 25’ Foliage: Ovate medium green leaves. Zone: 5 Bloom: Purple-red, chalice-shaped blooms; Foliage: Obovate large green leaves with slightly fragrant. hairs concentrated on the veins. Bloom Size: 4” Bloom: Fragrant creamy-yellow flowers with Bloom time:Mid-April to early May tinges of yellow-green near the base. Bark: Smooth tan or grey bark Bloom Size: 3” Growth Habit: Upright compact, shrubby Bloom Time: March to April before leaves habit; less apt to suffer frost damage in emerge. spring; slow growing. Bark: Smooth grey bark. Growth Habit: Pyramidal habit. MagnoliaBrixton campbellii Belle Magnolia'Brixton Belle' Height: 13’ MagnoliaEmerald virginiana Tower™ 'JN8' Magnolia Width: 11’ Zone: 5 Height: 20’ Foliage: Large oval green leaves. Width: 8’ Bloom: Large rich pink cup and saucer blooms; Zone: 5 flowers are frost resistant. Foliage: Shiny green foliage. Bloom Size: 6” Bloom: Fragrant, creamy-white blooms. Bloom time: Late winter, early spring. Bloom Size: 3” Bark: Beautiful smooth grey bark, similar to Bloom Time: Spring and summer against beech trees. large glossy leaves. Growth Habit: Upright shrub, small tree; often Bark: Smooth grey bark.