Extending Recombinant Factor IX Fc Fusion Protein Dosing Interval to 14 Or More Days in Patients with Hemophilia B
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Year of Investment, Innovation and Implementation
2017 the year of investment, innovation and implementation 2017 annual report healthcare businesswomen’s association table of contents LETTER FROM CHAIR 1 INVESTMENT 2017 BY THE NUMBERS 2 MEMBERSHIP 2 CHAPTER BREAKDOWN 2 40 YEARS OF MILESTONES 2 MEMBER/VOLUNTEER SURVEY RESULTS 3 FINANCIALS 4 INNOVATION AND IMPLEMENTATION 2017 FLAGSHIP EVENTS 7 WOMAN OF THE YEAR 8 LUMINARIES 10 RISING STARS 11 ANNUAL CONFERENCE 13 ACE AWARDS 14 THE GENDER PARITY COLLABORATIVE CLICK HERE HBA “NOW” THE NEW OPERATING MODEL CLICK HERE IN APPRECIATION—WE COULDN’T DO IT WITHOUT YOU 2017 CORPORATE PARTNERS 15 2017 SPONSORS 16 2017 MEDIA PARTNERS 17 HBA ADVISORY BOARD 18 HBA BOARD OF DIRECTORS 19 LETTER FROM CEO LAURIE COOKE 20 HBA 2017 II annual report letter from the chair In my long career as a healthcare executive, I have seen firsthand the benefits of diversity in leadership time and again. From gender to ethnicity to age and other factors, the best engagement and the most powerful results always arise when there is a diversity of perspectives in the room. This is why I joined the HBA board. This year marks the 40th anniversary of the organization’s founding. And though I am a relatively new member, I quickly became a believer in the HBA’s rich history and deep commitment to both the advancement of women individually, and to the achievement of gender parity in healthcare overall. This is also why I am proud of the HBA’s aggressive—but achievable—strategic plan to move the needle on gender parity through partnership by 2020. -

ANNUAL and SUSTAINABILITY REPORT 2017 Forward-Looking Statements This Report Includes Forward-Looking State- Ments
Building on our strengths Building ANNUAL AND SUSTAINABILITY REPORT 2017 Forward-looking statements This report includes forward-looking state- ments. Actual results may differ from those stated. Internal factors such as the successful management of research programmes and intellectual property rights may affect future results. There are also external conditions such as the economic climate, political changes and competing research programmes that may affect Sobi’s results. Sobi™ is an international biotechnology company dedicated to rare diseases. Our mission is to transform the lives of people with rare diseases by providing inno vative therapies in our focus areas.” Guido Oelkers, CEO CONTENTS Contents This is Sobi .......................................................................4 The year in brief ..........................................................6 INTERVIEW Interview with the CEO and Chairman ......................8 CEO and Chairman Strategy .....................................................................10 8 Sobi operates throughout the entire value chain, from early research, over to clinical development and into the commercial market. Business areas ...............................................................16 “We can add significant value to our partnerships by leveraging all Haemophilia ..............................................................16 our com petencies, making us the partner of choice for a range of Specialty Care ...........................................................22 companies -

Guidelines with Regard to the Composition, Calculation and Management of the Index
INDEX METHODOLOGY Solactive Pharma Breakthrough Value Index Version 2.1 dated September 03, 2020 Contents Important Information 1. Index specifications 1.1 Short Name and ISIN 1.2 Initial Value 1.3 Distribution 1.4 Prices and Calculation Frequency 1.5 Weighting 1.6 Index Committee 1.7 Publication 1.8 Historical Data 1.9 Licensing 2. Composition of the Index 2.1 Selection of the Index Components 2.2 Ordinary Adjustment 2.3 Extraordinary Adjustment 3. Calculation of the Index 3.1 Index Formula 3.2 Accuracy 3.3 Adjustments 3.4 Dividends and other Distributions 3.5 Corporate Actions 3.6 Correction Policy 3.7 Market Disruption 3.8 Consequences of an Extraordinary Event 4. Definitions 5. Appendix 5.1 Contact Details 5.2 Calculation of the Index – Change in Calculation Method 2 Important Information This document (“Index Methodology Document”) contains the underlying principles and regulations regarding the structure and the operating of the Solactive Pharma Breakthrough Value Index. Solactive AG shall make every effort to implement regulations. Solactive AG does not offer any explicit or tacit guarantee or assurance, neither pertaining to the results from the use of the Index nor the Index value at any certain point in time nor in any other respect. The Index is merely calculated and published by Solactive AG and it strives to the best of its ability to ensure the correctness of the calculation. There is no obligation for Solactive AG – irrespective of possible obligations to issuers – to advise third parties, including investors and/or financial intermediaries, of any errors in the Index. -

Investor Presentation March 19, 2019
Transaction Update INVESTOR PRESENTATION MARCH 19, 2019 1 Important Information For Investors And Stockholders This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval. It does not constitute a prospectus or prospectus equivalent document. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended. In connection with the proposed transaction between Bristol-Myers Squibb Company (“Bristol-Myers Squibb”) and Celgene Corporation (“Celgene”), on February 1, 2019, Bristol-Myers Squibb filed with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-4, as amended on February 1, 2019 and February 20, 2019, containing a joint proxy statement of Bristol-Myers Squibb and Celgene that also constitutes a prospectus of Bristol- Myers Squibb. The registration statement was declared effective by the SEC on February 22, 2019, and Bristol-Myers Squibb and Celgene commenced mailing the definitive joint proxy statement/prospectus to stockholders of Bristol-Myers Squibb and Celgene on or about February 22, 2019. INVESTORS AND SECURITY HOLDERS OF BRISTOL-MYERS SQUIBB AND CELGENE ARE URGED TO READ THE DEFINITIVE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION. Investors and security holders will be able to obtain free copies of the registration statement and the definitive joint proxy statement/prospectus and other documents filed with the SEC by Bristol-Myers Squibb or Celgene through the website maintained by the SEC at http://www.sec.gov. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

Specialty Pharmacy Product Access List – ARJ Infusion Services
Specialty Pharmacy Product Access List – ARJ Infusion Services ARJ Infusion Services' comprehensive specialty medication list contains nearly 200 products, covering over 15 therapeutic categories, and specialty disease states. *ARJ access to a limited drug distribution network Date last modified: January 12, 2021 Medication Specialty Manufacturer Xolair* Allergy and Asthma Genentech Nucala* Allergy and Asthma GSK Fasenra* Allergy and Asthma AstraZeneca Entyvio Chronic Inflammatory Takeda Enbrel Chronic Infammatory Amgen Dupixent Allergy and Asthma Sanof Genzyme Dupixent Chronic Infammatory Sanof Genzyme Cinqair* Allergy and Asthma Teva Benlysta* Chronic Infammatory GSK Avsola* Chronic Infammatory Amgen Actemra* Chronic Infammatory Genentech Humira Chronic Infammatory Abbvie Krystexxa* Gout Horizon Ilumya Chronic Infammatory Sun Pharma Infectra Chronic Infammatory Pfzer Linzess Chronic Infammatory Allergan Orencia Chronic Infammatory BMS Remicade Chronic Infammatory Janssen Renfexis Chronic Infammatory MERCK Rituxan* Chronic Infammatory Genentech Simponi Chronic Infammatory Janssen Simponi Aria Chronic Infammatory Janssen Skyrizi Chronic Infammatory Abbvie Stelara Chronic Infammatory Janssen Zinplava Chronic Infammatory MERCK Aldurazyme* Enzyme Defciency BioMarin Cerezyme* Enzyme Defciency Sanof Genzyme Elaprase* Enzyme Defciency Takeda Fabrazyme* Enzyme Defciency Sanof Genzyme Lumizyme Enzyme Defciency Sanof Genzyme Vpriv* Enzyme Defciency Takeda Omnitrope Growth Hormone Therapy Sandoz Sandostatin Growth Hormone Therapy Novartis Sandostatin -

Acquired Hemophilia a International Recommendations on the Diagnosis
Haematologica HAEMATOL/2019/230771 Version 5 Haematologica HAEMATOL/2019/230771 Version 5 International recommendations on the diagnosis and treatment of acquired hemophilia A Andreas Tiede, Peter Collins, Paul Knoebl, Jerome Teitel, Craig Kessler, Midori Shima, Giovanni Di Minno, Roseline d' Oiron, Peter Salaj, Victor Jiménez-Yuste, Angela Huth-Kühne, and Paul Giangrande Disclosures: AT has received consultancy, lecture fees or research grants from Alnylam, Bayer, Biogen Idec, Biotest, Chugai, CSL Behring, Novo Nordisk, Octapharma, Pfizer, Roche, Shire, and SOBI. PC has received consultancy, lecture fees for research grants from Bayer, Biogen Idec, Chugai, CSL Behring, Novo Nordisk, Roche and Sobi. PK has received consultancy, lecture fees or research grants from Biotest, CSL Behring, Novo Nordisk, Roche, Shire/Takeda. JT has received consultancy, lecture fees, or research support from Bayer, Takeda, Pfizer, Novo Nordisk, Roche, Octapharma, and Bioverativ. CK has served on advisory boards for Bayer, Novo Nordisk, Octapharma, Pfizer, Takeda, and Genentech and has received research funding from Bayer, NovoNordisk, Octapharma, Takeda, and Genentech. He serves on DSMBs for Bayer, Dimensions, Octapharma, and the National Institutes of Health. MS has received consultancy and lecture fees and research grants from Shire, Bayer, Novo Nordisk, CSL Behring, Bioverativ, Chugai. GDM has received honoraria for scientific activities from Bayer, Novo Nordisk, Pfizer, Kedrion, CSL Behring and Sanofi. RdO has received reimbursement for attending symposia/congresses and/or honoraria for lectures or consultancy fees from Bayer, Baxter/Baxalta/Shire, LFB, NovoNordisk, CSL Behring, Octapharma, Pfizer, Roche, Sobi and Spark. PS has received lecture fees from Novo Nordisk and Shire/Takeda. VJY has received reimbursement for attending symposia/congresses and/or honoraria for speaking and/or honoraria for consulting, and/or funds for research from Shire, Bayer, CSL-Behring, Grifols, Novo Nordisk, Sobi, Roche, Octapharma and Pfizer. -

Health Care Fund SCHEDULE of INVESTMENTS (Unaudited) September 30, 2017
Health Care Fund SCHEDULE OF INVESTMENTS (Unaudited) September 30, 2017 Shares Value COMMON STOCKS† - 99.8% Pharmaceuticals - 35.0% Johnson & Johnson 6,515 $ 847,014 Pfizer, Inc. 18,337 654,631 Merck & Company, Inc. 9,253 592,470 AbbVie, Inc. 5,629 500,193 Bristol-Myers Squibb Co. 7,228 460,713 Eli Lilly & Co. 5,197 444,551 Allergan plc 1,785 365,836 Shire plc ADR 1,949 298,470 Express Scripts Holding Co.* 4,418 279,748 McKesson Corp. 1,712 262,980 Zoetis, Inc. 3,926 250,322 Teva Pharmaceutical Industries Ltd. ADR 13,456 236,826 AstraZeneca plc ADR 6,378 216,087 Cardinal Health, Inc. 3,150 210,798 GlaxoSmithKline plc ADR 4,882 198,209 Jazz Pharmaceuticals plc* 1,351 197,584 AmerisourceBergen Corp. — Class A 2,384 197,276 Endo International plc* 23,012 197,098 Alkermes plc* 3,792 192,785 Novartis AG ADR 2,243 192,562 Perrigo Company plc 2,224 188,262 Novo Nordisk A/S ADR 3,749 180,514 Mylan N.V.* 5,703 178,903 TESARO, Inc.* 1,050 135,555 Neurocrine Biosciences, Inc.* 1,760 107,853 Valeant Pharmaceuticals International, Inc.* 7,404 106,099 PRA Health Sciences, Inc.* 1,370 104,353 Clovis Oncology, Inc.* 1,260 103,824 Premier, Inc. — Class A* 3,040 99,013 ACADIA Pharmaceuticals, Inc.* 2,558 96,360 Catalent, Inc.*,1 2,410 96,207 Mallinckrodt plc* 2,570 96,041 Akorn, Inc.* 2,823 93,695 Avexis, Inc.* 910 88,024 Nektar Therapeutics* 3,630 87,120 Portola Pharmaceuticals, Inc.* 1,530 82,666 DexCom, Inc.* 1,635 79,992 Agios Pharmaceuticals, Inc.* 1,176 78,498 Amicus Therapeutics, Inc.*,1 5,066 76,395 Prestige Brands Holdings, Inc.* 1,480 74,133 Horizon Pharma plc* 5,582 70,780 Sarepta Therapeutics, Inc.* 1,559 70,716 Radius Health, Inc.*,1 1,790 69,005 Ironwood Pharmaceuticals, Inc. -

The Scientific Organizing Committee Gratefully Acknowledges the Pharmaceutical and Biotechnology Industry for Their Generous Support of WCBP 2020
The Scientific Organizing Committee gratefully acknowledges the pharmaceutical and biotechnology industry for their generous support of WCBP 2020: Strategic Diamond Program Partners F. Hoffmann-La Roche Ltd. Genentech, a Member of the Roche Group Strategic Platinum Program Partners Amgen Inc. AstraZeneca Biogen Merck & Co., Inc. Strategic Gold Program Partners AbbVie Bioresearch Center, Inc. Eli Lilly and Company Novo Nordisk A/S Pfizer, Inc. Sanofi Strategic Silver Program Partner Bristol-Myers Squibb Company 1 Gold Program Partners Bill and Melinda Gates Foundation Jazz Pharmaceuticals Silver Program Partners BioMarin Pharmaceutical Inc. GlaxoSmithKline Seattle Genetics, Inc. Friends of CASSS Janssen Pharmaceutical R&D, LLC Omeros Corporation Seqirus, A CSL Company 2 The Scientific Organizing Committee gratefully acknowledges the program partners and exhibitors for their generous support of WCBP 2020: Diamond Program Partners Agilent Technologies, Inc. Catalent, Inc. ProteinSimple, a bio-techne brand SCIEX Thermo Fisher Scientific Waters Corporation Platinum Program Partners BioAnalytix, Inc. / Protagen Protein Services GmbH 3 The Scientific Organizing Committee gratefully acknowledges the exhibitors for their generous support of WCBP 2020: Exhibitor Partners Agilent Technologies, Inc. PerkinElmer Associates of Cape Cod, Inc. Postnova Analytics Inc. BioAnalytix, Inc. / Protagen Protein PPD Services GmbH Bruker BioSpin Corporation Pressure BioSciences Inc. Catalent, Inc. Protein Metrics, Inc. Charles River Laboratories ProteinSimple, -

Lsx C-Suite Challenges in Life Sciences Survey 2018 Lsx C-Suite Challenges in Life Sciences Survey 2018
LSX C-SUITE CHALLENGES IN LIFE SCIENCES SURVEY 2018 LSX C-SUITE CHALLENGES IN LIFE SCIENCES SURVEY 2018 LEAD SPONSOR’S COMMENT John Ratliff, CEO at Covance, Inc. Heraclitus, the Greek philosopher, What this research also makes clear, aligning with what we’re seeing from our clients every day, is that drug who said: “change is the only development is not a one-size-fits-all activity. Beyond the typical impediments to successful drug development, constant in life,” must have had a nimble biotech firms are requiring solutions that address their unique business challenges, particularly in the areas of premonition regarding the state funding, licensing and partnership. of the drug development industry The good news is that the market continues to sustain venture capitalist funding in the mid-$30bn range per year*. today. Most conspicuous is the However, challenges still remain around how to identify the right investment partners at the right time and how to accelerating amount of innovation effectively show the value of your asset to investors who within nimble biopharmaceutical may not have the scientific knowledge or appetite for risk. For those with the goal of bringing their drug through organisations that are now driving IND/CTA submission or first-in-human and finding a pharmaceutical partner to license or co-develop their asset, nearly three quarters of the active the challenges are similar – being visible in the right place at pipeline. Small seems to be the new the right time and finding a strategic, cultural fit. Staying focused and managing lean resources also requires big – and biotechs are doing it with the need to partner strategically with early development and fresh passion and perseverance to clinical research partners – with the lion’s share of biotechs doing their drug development alongside a CRO partner. -
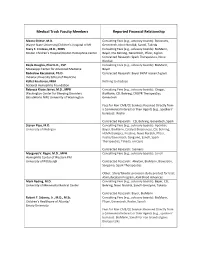
To Review All Faculty Disclosures, Click Here
Medical Track Faculty Members Reported Financial Relationship Meera Chitlur, M.D. Consulting Fees (e.g., advisory boards): Bioverativ, Wayne State University/Children's Hospital of MI Genentech, Novo Nordisk, Sanofi, Takeda Stacy E. Croteau, M.D., MMS Consulting Fees (e.g., advisory boards): BioMarin, Boston Children's Hospital/Boston Hemophilia Center Bayer, CSL Behring, Genentech, Pfizer, Sigilon Contracted Research: Spark Therapeutics, Novo Nordisk Kayla Douglas, Pharm.D., CSP Consulting Fees (e.g., advisory boards): BioMarin, Mississippi Center for Advanced Medicine Bayer Radoslaw Kaczmarek, Ph.D. Contracted Research: Bayer BHAP research grant Indiana University School of Medicine Kollet Koulianos, MBA Nothing to disclose National Hemophilia Foundation Rebecca Kruse-Jarres, M.D., MPH Consulting Fees (e.g., advisory boards): Chugai, Washington Center for Bleeding Disorders BioMarin, CSL Behring, CRISPR Therapeutics, BloodWorks NW/ University of Washington Genentech Fees for Non-CME/CE Services Received Directly from a Commercial Interest or their Agents (e.g., speakers’ bureaus): Roche Contracted Research: CSL Behring, Genentech, Spark Steven Pipe, M.D. Consulting Fees (e.g., advisory boards): Apcintex, University of Michigan Bayer, BioMarin, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline, Novo Nordisk, Pfizer, Roche/Genentech, Sangamo, Sanofi, Spark Therapeutics, Takeda, uniQure Contracted Research: Siemens Margaret V. Ragni, M.D., MPH Consulting Fees (e.g., advisory boards): Sanofi Hemophilia Center of Western PA/ University of Pittsburgh Contracted Research: Alnylam, BioMarin, Bioverativ, Sangamo, Spark Therapeutics Other: Shire/Takeda: provision study product for trial; ASH Education Program, ASH Blood Advances Mark Reding, M.D. Consulting Fees (e.g., advisory boards): Bayer, CSL University of Minnesota Medical Center Behring, Novo Nordisk, Sanofi-Genzyme, Takeda Contracted Research: Bayer, BioMarin Robert F. -

Partners; Kinneret Livnat Savitzky, Interim
PEOPLE Gene therapy developer Prevail Therapeutics (New York) has where he served in the same position. He also announced the appointment of Francois Nader (left) to its board served as chief commercial officer at ChanTest, of directors. Nader was president, CEO and executive director now part of Charles River Laboratories. of NPS Pharma from 2008 until 2015, when the company was acquired by Shire. Before joining NPS Pharma, he was a venture Amit Patel has been elected to the board of direc- partner at Care Capital. tors of privately held Calyptus Pharmaceuticals “I am honored to join the board of Prevail Therapeutics,” (Princeton, NJ, USA). Patel has 20 years of said Nader. “The Prevail team has already made great progress healthcare industry experience, most recently in developing potential treatments for Parkinson’s disease and serving as senior vice president and president other neurodegenerative diseases, and I look forward to partnering with the team to help at Capsugel. Prior to Capsugel, he worked at Dr. accelerate important therapeutic advances for patients with these devastating illnesses.” Reddy’s Laboratories, where he held the posi- tions of executive vice president & head, North Gastrointestinal disease treatment developer CEO of Immunocore, as non-executive chair- America, and senior vice president & head, global Ritter Pharmaceuticals (Los Angeles) has man of the board, succeeding Trevor Nichols, corporate development & strategic planning. appointed John W. Beck as CFO. He served in who has acted as chairman since January 2014. the same position at Ardea Biosciences before Nichols will remain on the board as a non- Yaping Shou has been appointed chief medi- it was acquired by AstraZeneca.