Aerobic Bacterial Isolates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

11 HS 000 ETH 013013 A4.Pdf (English)
ETHIOPIA:Humanitarian Concern Areas Map (as of 04 February 2013) Eritrea > !ª !ª> Note: The following newly created woreda boundaries are not Tahtay !ª E available in the geo-database; hence not represented in this Nutrition Hotspot Priority Laelay Erob R R !ª Adiyabo Mereb Ahferom !ª Tahtay Gulomekeda !ª I E map regardless of their nutrition hot spot priority 1 & 2: Adiyabo Leke T D Adiyabo Adwa Saesie Dalul Priority one Asgede Tahtay R S Kafta Werei Tsaedaemba E E Priority 1: Dawa Sarar (Bale zone), Goro Dola (Guji zone), Abichu Tsimbila Maychew !ª A Humera Leke Hawzen Berahle A Niya( North Showa zone) and Burka Dintu (West Hararge Priority two > T I GR AY > Koneba Central Berahle zone) of Oromia region, Mekoy (Nuer zone) of Gambella Western Naeder Kola Ke>lete Awelallo Priority three Tselemti Adet Temben region, Kersadula and Raso (Afder zone), Ararso, Birkod, Tanqua > Enderta !ª Daror and Yo'ale (Degahabour zone), Kubi (Fik zone), Addi Tselemt Zone 2 No Priority given Arekay Abergele Southern Ab Ala Afdera Mersin (Korahe zone), Dhekasuftu and Mubarek (Liben Beyeda Saharti Erebti Debark Hintalo !ª zone), Hadigala (Shinille zone) and Daratole (Warder Abergele Samre > Megale Erebti Bidu Wejirat zone) of Somali region. Dabat Janamora > Bidu International Boundary Alaje Raya North Lay Sahla Azebo > Wegera Endamehoni > > Priority 2: Saba Boru (Guji zone) of Oromia region and Ber'ano Regional Boundary Gonder Armacho Ziquala > A FA R !ª East Sekota Raya Yalo Teru (Gode zone) and Tulu Guled (Jijiga zone) of Somali region. Ofla Kurri Belesa -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -
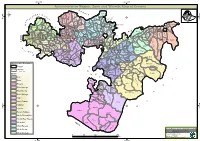
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

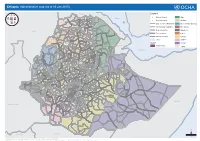
Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

Ethiopia Administrative Map As of 2013
(as of 27 March 2013) ETHIOPIA:Administrative Map R E Legend E R I T R E A North D Western \( Erob \ Tahtay Laelay National Capital Mereb Ahferom Gulomekeda Adiyabo Adiyabo Leke Central Ganta S Dalul P Afeshum Saesie Tahtay Laelay Adwa E P Tahtay Tsaedaemba Regional Capital Kafta Maychew Maychew Koraro Humera Asgede Werei Eastern A Leke Hawzen Tsimbila Medebay Koneba Zana Kelete Berahle Western Atsbi International Boundary Welkait Awelallo Naeder Tigray Wenberta Tselemti Adet Kola Degua Tsegede Temben Mekele Temben P Zone 2 Undetermined Boundary Addi Tselemt Tanqua Afdera Abergele Enderta Arekay Ab Ala Tsegede Beyeda Mirab Armacho Debark Hintalo Abergele Saharti Erebti Regional Boundary Wejirat Tach Samre Megale Bidu Armacho Dabat Janamora Alaje Lay Sahla Zonal Boundary Armacho Wegera Southern Ziquala Metema Sekota Endamehoni Raya S U D A N North Wag Azebo Chilga Yalo Amhara East Ofla Teru Woreda Boundary Gonder West Belesa Himra Kurri Gonder Dehana Dembia Belesa Zuria Gaz Alamata Zone 4 Quara Gibla Elidar Takusa I Libo Ebenat Gulina Lake Kemkem Bugna Kobo Awra Afar T Lake Tana Lasta Gidan (Ayna) Zone 1 0 50 100 200 km Alfa Ewa U Fogera North Farta Lay Semera ¹ Meket Guba Lafto Semen Gayint Wollo P O Dubti Jawi Achefer Bahir Dar East Tach Wadla Habru Chifra B G U L F O F A D E N Delanta Aysaita Creation date:27 Mar.2013 P Dera Esite Gayint I Debub Bahirdar Ambasel Dawunt Worebabu Map Doc Name:21_ADM_000_ETH_032713_A0 Achefer Zuria West Thehulederie J Dangura Simada Tenta Sources:CSA (2007 population census purpose) and Field Pawe Mecha -

Download Map.Pdf
ETHIOPIA: Hot Spot Map (as of 21 November 2011) Eritrea !ª !ª E Legend Tahtay Laelay !ª Erob R R !ª Mereb Gulomekeda !ª !ª Adiyabo Adiyabo I E Leke Ahferom Dalul T D International Boundary > North >Adwa> > Saesie S Tsaedaemba N R Kafta WesternN Central B E Humera B Werei !ª E A International Boundary Medebay > Hawzen Koneba TI G RAY Leke Eastern Berahle A Western Zana N N Kola Degua B B W Regional Boundary Tselemti Temben Kelete Temben Awelallo > W Tselemt Enderta Zone 2 !ª Zonal Boundary Saharti Afdera Mirab Beyeda Samre Hintalo Ab Ala > Abergele Wejirat !ª Armacho N Megale Erebti Lakes Tach B Wag W W N Southern Bidu Armacho JanamoraBN HimraB Alaje W > N W Hot Spot Areas Sahla N > > B North Wegera B Endamehoni Metema Ziquala Sekota AFA R !ª N Ó No Data/No Humanitarian Concern Gonder d N Raya Yalo Teru dÓWest BEast Ofla B Azebo N Kurri Gonder Belesa Belesa Dehana N B N W Gaz Alamata B B W Dembia Zuria N N Zone 4 > Elidar Critical Quara B GiblBa Libo N I Ebenat BBugna Lasta Gulina Awra N S U D A N Kemkem Lay B N (Ayna) Gidan > T Medium Humanitarian Concern BGayint BN Fogera North Ewa U Farta Guba N N Bahirdar Meket Semen Wollo LaftoB B O G ULF O F ADEN Close Monitoring Zuria Dubti Achefer South Wadla Chifra West Delanta Aysaita B West Gonder Tach N Zone 1 I dÓ Esite B Ambasel Worebabu Guba Gojam Gayint W J Hot Spot Indicators -- This month AM HAR A Simada Oromia Adaa'r Mile Mekdela Afambo D Metekel East Bati W W Sayint > Water Shortage/WASH related concerns Sirba Esite Special Sudanese Refugees Abay N South Zone UNHCR report indicates 19,800 Awi/Agew BWollo Woreda 5 W N Sudanese refugees in Benishangul 7 N Chefa Dewe W N Food Insecurity B High Malnutrition Ó Sherkole BE N IS HAN GU L B B Ayisha region as of 16 November 2011. -

Annual Report International Organization for Migration Special Liaison Office (IOM SLO) in Addis Ababa, Ethiopia
2015Annual Report International Organization for Migration Special Liaison Office (IOM SLO) in Addis Ababa, Ethiopia IOM OIM IOM PRESENCE In EthIOpIA IOM Presence in Ethiopia ETHIOPIA: Administrative Map (as of 14 January 2011) R ShireERITREA E Legend Tahtay Erob Laelay Adiyabo Mereb Ahferom Gulomekeda \\( Adiyabo Leke D National Capital Ganta Medebay Dalul North Adwa Afeshum Saesie Tahtay Zana Laelay Tsaedaemba Kafta Western Maychew PP Koraro Central Humera Asgede Tahtay Eastern Regional Capital Naeder Werei Hawzen Western Tsimbila Maychew Adet Leke Koneba Berahle Welkait Kelete Atsbi S Tigray Awelallo Wenberta International Boundary Tselemti Kola Degua Tsegede Mekele E Temben Temben P Addi Tselemt Tanqua Afdera Zone 2 Enderta Arekay Abergele Regional Boundary Tsegede Beyeda Ab Ala Mirab Saharti A Armacho Debark Samre Hintalo Erebti Abergele Wejirat Tach Megale Bidu Zonal Boundary Armacho Dabat Janamora Alaje Lay Sahla North Armacho Wegera Southern Ziquala Woreda Boundary Metema Gonder Sekota Endamehoni Raya Wag Azebo Chilga Yalo Amhara East Ofla Teru West Belesa Himra Kurri Gonder Dehana Belesa Lake Dembia Zuria Gaz Alamata Zone 4 Quara Gibla Semera Elidar Takusa Libo Ebenat Gulina Kemkem Bugna Lasta Kobo Awra Afar Gidan Lake Tana South (Ayna) 0 50 100 200 km Ewa Alfa Fogera Gonder North ¹ Lay Zone 1 Farta Meket Guba Lafto Dubti Gayint Asayta Semen Wollo P Jawi Achefer Tach Habru Chifra Bahr Dar East Wadla Delanta G U L F O F A D E N P Gayint Aysaita Creation date:14 Jan.2011 Dera Esite Bahirdar Ambasel Map Doc Name:21_ADM_000_ETH_011411_A0 -

OROMIA REGION : Who Does What Where (3W) - WASH Sector (As of 28 February 2013)
OROMIA REGION : Who Does What Where (3W) - WASH Sector (as of 28 February 2013) Tigray Beneshangul Amhara Afar Amhara Gumu HEKS:k Christian Aid:k Afar Beneshangul Gumu Dire Dawa Addis Ababa Hareri Dera Amuru Gambela Oromia Ibantu CRS: Hidabu WVE: Plan Int.: CRS: Somali Gida k k WVE: CISP: SNNPR Kiremu Jarte Wara Abote Save the k ECS:k k Jarso Degem Mercy Corps: Haro East Jardega North Childern:k Horo Ababo Shewa(R4) Gerar Mana Kiltu Limu Wellega WVE: Abuna Jarso De! bre CARE: CRS: Sibu Kara Limu Guduru Kuyu WVE: Ginde Libanos Abichuna k ! Abe Horo Abay G/Beret Dire Dawa WVE: Yaya! West k k Beret Gne'a ECS: k Chinaksen Dongoro ! Wuchale Nejo ! Chomen ! Gulele Jarso ! Wellega Jimma Meta ! Haro ! Guduru ! Adda Goro Kombolcha Gudetu Jarso Guto Genete Robi Jida Maya ! Babo Boji Bila Kembibit Gutu Meta Kondole Boji Gida Berga ! Kersa ! Gursum ! Sululta Dirmeji Seyo Jeldu Mulo Doba Kurfa Jimma Aleltu ! Begi Chekorsa Deder Lalo Sasiga Gobu Rare Mida West Tulo Chele Harari CRS: CARE: Ayira ! Gawo Gimbi Ifata Mieso ! Asabi Seyo Kegn Shewa Ejere Bereh Kebe Guliso Bako Ambo (Addis Chiro WVE:k k Sibu Malka ! ECS: Gaji Wayu Tibe Cheliya Zuria Bedeno Fedis Jimma Dale Diga Sire Toke Dendi Alem) Addis Zuria ! Haru Tuka Gimbichu Mesela Balo Girawa Horo Wabera Yubdo Boneya Kutaye Goba Chwaka Leka ! Walmara WVE: WFeVntEal:e Gidami Lalo Save the Tikur ! k k Koricha Gemechis Dulecha Wama Boshe Ilu Ababa Yama Logi Dale Nole Sayo Dawo Alem ! Kile Childern:k Enchini Akaki Midega Babile CRS: Kelem Dabo Hagalo ! Ada'a ! Habro Welel Sadi South ! Kaba Nole Meko Dano -

OROMIA REGION : Who Does What Where (3W) - Health and Nutrition E N U D L F O F a Tigray Amhara Afar Afar Amhara
(as of 15 August 2012) G N OROMIA REGION : Who Does What Where (3W) - Health and Nutrition E U D L F O F A Tigray Amhara Afar Afar Amhara Benshangul Gumuz Dire Dawa Addis Ababa Hareri Beneshangul Gumuz Gambela SC US: Oromia f CARE: f Somali SNNPR WVE: f Dare WVE: h WVE: ECS: f f CARE: f Hidabu WVE: GOAL: GOAL: h CARE: h ERCS: f SC US: h Abote f WVE: h Horo Guduru h ECS: f ERCS: f Kuyu WVE: f WVE: f SC US: h GOAL: h Ginde Beret North Shewa(R4) GOAL: h Chinaksen Horo Abichuna ECS: f Nejo Meta Wuchale CARE: fh Jarso West Wellega SC US: f SC US: f Adda Gne'a ERCS:f Jimma Guduru Robi Kembibit CARE: f Jeldu Berga Meta Kersa IMC: h Genete WVE: h SC US:f Doba Kurfa Gursum WVE: f Lalo East Wellega Ejere (Addis Alem) Aleltu Deder CARE: h Sibu Mida Kegn WVE: f Mieso Tulo Chele Asabi WVE: f Bako West Shewa Bereh ECS: f Sire Ambo WalmaraAddis SC US:f Chiro Zuria Girawa Fedis Tibe Dendi Gimbichu Bedeno IMC: h Cheliya Zuria ASbCab UaS: h WVE: Malka f Fentale Gemechis East Harerge Kelem Wellega SC US: fh Ilu Balo Midega Tikur Dawo SC US: f SC US: f DanoJibat Ada'a Anchar Habro WVE: Tola ERBCaSb:i le Enchini Wenchi Becho Boset f f Tole Lome WVE: CARE: Nono Ameya Waliso Kondaltiti h Daro fh Ilu Aba Bora Badele SC US: East Shewa West Harerge ECS: f South West Shewa Aseko Lebu f Zuria PlaAnd aInmt.a: Jeju Boke CARE: fh Bora h Gololcha Gechi SC US: WVE: Meyu WVE: f SC US:f fh f Arsi ERCS: f WVE: f Sekoru Dugda Chole GOAL: fh CRS: f SC US: fLude Hitosa IMC: h Arsi ECS: f GumayJimma SC US: f ATJK Sude CRS: h ERCS: f Tiyo WVE: h Legend Gambella GomaMena Sekoru -

Pact Inc. in Zambia and Ethiopia the Y-CHOICES Program Annual Report
Pact Inc. in Zambia and Ethiopia The Y-CHOICES Program Cooperative Agreement No. GPO-A-00-04-00024-00 Annual Report October 1, 2005 – September 30, 2006 Submitted: November 8, 2006 Program Duration: October 1, 2004 – September 29, 2009 Table of Contents Acronyms…………………..……………………………………………………………………2 I. Executive Summary…………………………………………………………………………..4 Strategic Objectives.....................................................................................................................4 General Overview of Activities and Approaches.........................................................................4 General Summary of Results and Successes ................................................................................5 Coming Six-months Activities.....................................................................................................6 II. Emergency Plan Indicators Tables…………………………………………………………7 Progress on Yearly Targets for Required Emergency Plan Indicators – Zambia and Ethiopia .....7 L.O.A. Progress Tracking Table for Emergency Plan Indicators –Zambia and Ethiopia .............9 III. Country-level Progress Report………………………………………………………..10 Pact Zambia Country Overview................................................................................................10 Pact Ethiopia - Country Overview ............................................................................................16 Ethiopia Y-CHOICES Operational Regions and Implementing Partners ...........................................16 Zambia - Summary of -
Ethiopia: Administrative Map (As of 05 Jan 2015)
Ethiopia: Administrative map (as of 05 Jan 2015) ERITREA Legend Ahferom Erob ^! Tahtay Adiyabo National Capital Gulomekeda Afar Laelay Adiyabo Mereb Leke Red Sea Dalul Ganta Afeshum P! Adwa SaesEiea Tsatedranemba Regional Capital Amhara North WesternTahtay KoraroLaelay Maychew Kafta Humera Tahtay Maychew Werei Leke Hawzen Asgede Tsimbila Central Koneba TIGRAY Medebay Zana Naeder Adet Berahile Western Atsbi Wenberta Undetermined boundary Beneshangul Gumuz Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele P! Zone 2 International boundary Dire Dawa Tsegede Tselemt Enderta Tanqua Abergele Afdera Addi Arekay Ab Ala Tsegede Beyeda Mirab Armacho Afdera Region boundary Gambela Debark Saharti Samre Erebti Hintalo Wejirat SUDAN Abergele Tach Armacho Dabat Janamora Megale Bidu Alaje Sahla Southern Zone boundary Hareri Ziquala Raya Azebo Metema Lay Armacho Wegera Wag Himra Endamehoni North Gonder Sekota Teru Chilga Woreda boundary Oromia Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Zone 4 Elidar Gaz Gibla Lake SNNPR Quara Takusa Ebenat Gulina Libo Kemkem Bugna Awra Kobo Tana Gidan Region Lasta (Ayna) AFAR Somali Alfa Ewa Fogera Farta North Wollo Semera Lay Gayint Meket Guba Lafto P! Dubti Zone 1 DJIBOUTI Addis Ababa Jawi Semen Achefer South Gonder Tigray East Esite Chifra Bahir Dar Wadla Habru Aysaita AMHARA P! Dera Tach Gayint Delanta Guba Bahirdar Zuria Ambasel Debub Achefer Dawunt Worebabu Dangura Gulf of Aden Mecha West Esite Simada Thehulederie Adaa'r Mile Pawe Special Afambo Dangila Kutaber Yilmana