Soil Health and Organic Farming Understanding and Optimizing the Community of Soil Life
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bokashi! 5/12/2014 Bokashi Fermentation Starter for Recycling Food Waste
bokashi! 5/12/2014 Bokashi fermentation starter for recycling food waste Home Use Instructions The bokashi* is usually a dry organic material that can be made with different ingredients for different uses. The following is one standard ingredients used to make bokashi (U.S. version). Ingredients: Wheat Bran**, Blackstrap Molasses, EM•1 Microbial Inoculant†, Water (ingredients are mixed, fermented and dried). Keep dry. The bokashi can be made at home or garden, or provided by a garden, or it can be purchased ready-to-use. * For more about bokashi and other uses, see page 5. ** Other organic materials can be used instead of wheat bran to house the microorganisms, including rice bran, dried leaves, dry coffee husk/chaff, dried brewery waste, saw dust/wood shavings (walnut, teak, pine —other kinds of wood may be difficult to properly ferment into bokashi), etc. † In the U.S., EM•1 Microbial Inoculant or EM•1 Waste Treatment can be used to make bokashi. The bokashi method of recycling food waste is a two-step process of converting all types of food waste back into the soil. Step 1: "pickle" or ferment the food waste to prepare it for Step 2: adding to soil. The method uses bokashi to sprinkle on food waste in an air-tight container in order so that the food waste will ferment instead of rot. Note. You do not need to use bokashi if you ferment only fruit and vegetable food scraps. However, it may take time to ferment, and it may not be consistent. By adding bokashi, you can more consistently get the food waste to ferment and to ferment more quickly, and also all food waste can then be recycled, including meats, dairy, bones, etc. -

Finca+Slow+Permaculture.Pdf
Farming and Smallholding © Johanna McTiernan Dan McTiernan describes how regenerative agriculture is transforming olive groves in Spain and introduces © Johanna McTiernan transnational cropshare Restoring Agriculture in the Mediterranean “It’s not just that traditional Mediter- Together with our friends, who own healthy, perennial Mediterranean crops heavy input, bare-earth paradigm ranean agriculture isn’t sustainable a similar piece of land, and working that can’t be grown in Britain easily. of agriculture that is having such a ... it isn’t even viable on any level in partnership with IPM, we have If managed holistically, olives, nut destructive impact on the environ- anymore!” That was one of the first started Terra CSA, a multi-farm com- bearing trees such as almonds, and ment and the climate. All other things Richard Wade of Instituto munity supported agriculture project vine products like red wine, are about non-cold-pressed seed oils require Permacultura Montsant (IPM) said using permaculture and regenerative as perennial and sustainable as crops high levels of processing involving to us during our six month intern- agriculture to build soil and deliver come. We want the UK to still be heat and solvents in the extraction ship with him here in the south of olive oil, almonds and wine direct to able to access these incredibly process that are energy and resource Catalunya, Spain. cropshare members in the UK. nutritious products alongside the heavy and questionable in terms of With his doom laden words still Having been involved in community need to relocalise as much of our health to people and the planet. -
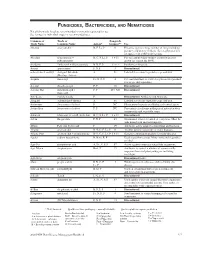
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

A Sustainable Agricultural Future Relies on the Transition to Organic Agroecological Pest Management
sustainability Review A Sustainable Agricultural Future Relies on the Transition to Organic Agroecological Pest Management Lauren Brzozowski 1 and Michael Mazourek 1,2,* ID 1 Section of Plant Breeding and Genetics, School of Integrative Plant Science, Cornell University, Ithaca, NY 14853, USA; [email protected] 2 David R. Atkinson Center for Sustainable Future, Cornell University, Ithaca, NY 14853, USA * Correspondence: [email protected] Received: 21 April 2018; Accepted: 11 June 2018; Published: 15 June 2018 Abstract: The need to improve agricultural sustainability to secure yields, minimize environmental impacts and buffer environmental change is widely recognized. Investment in conventional agriculture has supported its present yield advantage. However, organic agriculture with agroecological management has nascent capacity for sustainable production and for increasing yields in the future. Conventional systems have leveraged reductionist approaches to address pests, primarily through pesticides that seek to eliminate biological factors that reduce yield, but come at a cost to human and ecosystem health, and leave production systems vulnerable to the development of pest resistance to these chemicals or traits. Alternatives are needed, and are found in organic production approaches. Although both organic and agroecology approaches encompass more than pest management, this aspect is a pivotal element of our agricultural future. Through increased investment and application of emerging analytical approaches to improve plant breeding for and management of these systems, yields and resilience will surpass approaches that address components alone. Keywords: organic agriculture; agroecology; pest management; plant breeding; biodiversity; sustainability; host plant resistance; pesticides 1. Achieving Needs for Agricultural Productivity and Pest Management Sustainably There is broad recognition among agricultural scientists that a growing world population will consume greater amounts of food and fiber with fewer resources available for production [1]. -

Northeast Organic Farming Association/ Massachusetts Chapter
September 2013 Newsletter Northeast Organic Farming Association/ Massachusetts Chapter Inside this Issue: On Gardening & Proposed Food Safety Regs Big Ag Tries to Preempt Teaching Stir Farmer Anger State Laws on Farm Practices page 14 page 16 page 20 From the Editor Northeast Organic Farming Association/ Massachusetts Chapter, Inc. By Nicole Belanger 411 Sheldon Road Barre, MA 01005 NOFA/Mass Public Relations 978-355-2853 (p) 978-355-4046 (f) Coordinator [email protected] www.nofamass.org As the peaches ripen and bit by bit the days get shorter, our thoughts turn to how we can store NOFA/Mass Board Meetings are open to all the summer’s abundance and extend the season. members. For more information please contact: Reflecting on what we might do differently next time, Executive Director, Julie Rawson we are also reminded of what we’ve learned over the [email protected] years. 978-355-2853 Longtime gardener and NOFA/Mass member Christie Higginbottom grew up gardening with her parents © 2002-2013 NOFA/Massachusetts in central MA. She shared those experiences as NOFA/Massachusetts is a 501 (c) (3) non-profit an educator and gained new ones working at Old organization. Contributions are tax-deductible to Sturbridge Village for many years. In this issue, the extent allowed by law. Christie tells us her unique story and all about her tried and true tips for preparing the garden for the cold. Not a member yet? CLICK HERE NOFA/Mass Executive Director and longtime organic farmer Julie Rawson also reflects on a rekindled respect for comfrey and its diverse uses. -

Arbuscular Mycorrhizal Fungi and Soil Aggregation
R.C.Suelo Nutr. Veg. 8 (2) 2008 (9-18) J. Soil Sc. Plant Nutr. 8 (2) 2008 (9-18) 9 ARBUSCULAR MYCORRHIZAL FUNGI AND SOIL AGGREGATION Fernando Borie, Rosa Rubio, Alfredo Morales Universidad de La Frontera. Casilla 54-D-Temuco. Corresponding author: [email protected] Hongos micorrícicos arbusculares y agregación de suelo Keywords: arbuscular mycorrhizal fungi, soil aggregates, glomalin. ABSTRACT Soil aggregation is governed by several biotic and abiotic components including land- use management. Aggregation is essential to maintain soil physical properties and facilitate biogeochemical cycling. Hyphae of arbuscular mycorrhizal fungi (AMF) are considered to be primary soil aggregators and there is a positively correlation between AMF hyphae and aggregate stability in natural systems. Recent evidence suggests that glomalin (GRSP), a glycoprotein produced by AMF hyphae which has a cementing capacity to maintain soil particles together, is mainly involved in such aggregation. However, recently controversial results together with reported shortcoming in glomalin determinat suggest to proceed with caution when studying glomalin in connection with soil aggregation. Relationships between glomalin and soil aggregates found in Chilean soils are discussed. 10 Arbuscular mycorrhizal fungi, Borie et al. Palabras Claves: Hongos micorrícicos, agregados de suelo, glomalina. RESUMEN La agregación de suelo es gobernada por una serie de factores bióticos y abióticos incluyendo el manejo del suelo. La agregación es fundamental para mantener las propiedades físicas del suelo y facilitar los ciclos biogeoquímicos. Las hifas de los hongos formadores de micorrizas arbusculares (MA) son consideradas como importantes agentes aglutinadores de partículas del suelo y se han descrito correlaciones positivas entre hifas de hongos MA y estabilidad de agregados en sistemas naturales. -

(Hardpan) for Precision Agriculture on So
SITE - SPECIFIC CHARACTERIZATION, MODELING AND SPATIAL ANALYSIS OF SUB-SOIL COMPACTION (HARDPAN) FOR PRECISION AGRICULTURE ON SOUTHEASTERN US SOILS by MEHARI ZEWDE TEKESTE (Under the Direction of Randy L. Raper and Ernest W. Tollner) ABSTRACT Natural and machinery traffic-induced subsoil compacted layers (soil hardpans) that are found in many southeastern US soils limit root growth with detrimental effects on crop productivity and the environment. Due to the spatial variability of hardpans, tillage management systems that use site-specific depth tillage applications may reduce fuel consumption compared to the conventional uniform depth tillage. The success of site-specific tillage or variable depth tillage depends on an accurate sensing of the hardpan layers, field positioning, and controlling the application of real-time or prescribed tillage. The over arching objective of the work was to understand and advance the art and science of soil compaction analysis and prediction with an eye towards compaction management in precision farming. The specific objectives were (1) To investigate the influences of soil parameters (soil moisture and bulk density), and the soil-cone frictional property on the interpretations of cone penetrometer data in predicting the magnitude and depth of hardpans, (2) To determine spatial variability for creating hardpan maps and (3) To investigate a passive acoustic based real-time soil compaction sensing method. The soil cone penetration problems were also modeled using finite element modeling to investigate the soil deformation patterns and evaluate the capability of the finite element method to predict the magnitude and depth of the hardpan. Laboratory experiments in a soil column study indicated that the soil cone penetration resistances were affected by soil moisture, bulk density and cone material type. -

Efficacy of Intercropping Pattern in Reducing Weeds Infestation in Okra, Maize and Pepper Intercrop
International Journal of Weed Science and Technology ISSN 4825-3499 Vol. 2 (1), pp. 063-069, January, 2018. Available online at www.advancedscholarsjournals.org © Advanced Scholars Journals Full Length Research Paper Efficacy of intercropping pattern in reducing weeds infestation in okra, maize and pepper intercrop *Ubini C. Thomas, Jaymiwhie Obanna and Ikogho B. Patrick Department of Crop and Soil Science, University of Port Harcourt, P. M. B 5323 Port Harcourt, Nigeria. Accepted 15 January, 2018 Field study was conducted to evaluate the efficacy of intercropping pattern in reducing weed infestation in okra, maize and pepper intercrop; at the teaching and research farm of Rivers State University of Science and Technology Port Harcourt, Nigeria during 2009 and 2010 cropping season. Three intercropping pattern namely; alternate row intercropping, strip row intercropping and mixed intercropping were compared to sole cropping in a randomized complete block design replicated three times. The result reveal that weed biomass were significantly lower in both years in all forms of intercropping pattern compared to sole cropping or mono-cropping. Weed smothering efficiency in both years showed that mixed pattern (45.7%) >alternate row pattern (33.4%) > strip row pattern (11.5%). Crop yield were better in an intercrop system for maize and pepper in both years compared to -1 sole crop. However, mean okra fruit yield was highest in sole cropping (3253 kg ha ) when compared -1 to intercropping pattern. Maize yield was highest in mixed pattern (8,987 kg ha ) and lowest in sole -1 -1 cropping (6,955 kg ha ) while pepper fruit yield was highest in strip row pattern (5,435 kg ha ) and -1 lowest in mixed pattern (1,562 kg ha ). -

AFLP Fingerprinting for Identification of Infra-Species Groups of Rhizoctonia Solani and Waitea Circinata Bimal S
atholog P y & nt a M l i P c r Journal of f o o b l i a o Amaradasa et al., J Plant Pathol Microb 2015, 6:3 l n o r g u y DOI: 10.4172/2157-7471.1000262 o J Plant Pathology & Microbiology ISSN: 2157-7471 Research Article Open Access AFLP Fingerprinting for Identification of Infra-Species Groups of Rhizoctonia solani and Waitea circinata Bimal S. Amaradasa1*, Dilip Lakshman2 and Keenan Amundsen3 1Department of Plant Pathology, University of Nebraska-Lincoln, Lincoln, NE 68583, USA 2Floral and Nursery Plants Research Unit and the Sustainable Agricultural Systems Lab, Beltsville Agricultural Research Center-West, Beltsville, MD 20705, USA 3Department of Agronomy and Horticulture, University of Nebraska-Lincoln, Lincoln, NE 68583 USA Abstract Patch diseases caused by Thanatephorus cucumeris (Frank) Donk and Waitea circinata Warcup and Talbot varieties (anamorphs: Rhizoctonia species) pose a serious threat to successful maintenance of several important turfgrass species. Reliance on field symptoms to identify Rhizoctonia causal agents can be difficult and misleading. Different Rhizoctonia species and Anastomosis Groups (AGs) vary in sensitivity to commonly applied fungicides and they also have different temperature ranges conducive for causing disease. Thus correct identification of the causal pathogen is important to predict disease progression and make future disease management decisions. Grouping Rhizoctonia species by anastomosis reactions is difficult and time consuming. Identification of Rhizoctonia isolates by sequencing Internal Transcribed Spacer (ITS) region can be cost prohibitive. Some Rhizoctonia isolates are difficult to sequence due to polymorphism of the ITS region. Amplified Fragment Length Polymorphism (AFLP) is a reliable and cost effective fingerprinting method for investigating genetic diversity of many organisms. -

HOW to IMPROVE SOIL DRAINAGE Charlotte Germane, Nevada County Master Gardener
GOT COMPACTION? HOW TO IMPROVE SOIL DRAINAGE Charlotte Germane, Nevada County Master Gardener From The Curious Gardener, Summer 2011 Do you suspect you might have a drainage problem in your garden? If your soil does not drain quickly enough, your plants will drown. Soils in the foothills Your soil drainage may not be as bad as you think it is. There’s so much talk in the foothills about clay soil that some gardeners assume they have poorly draining soil, and grumble about it, when they actually have pretty respectable loam. The USDA has mapped soil types and found that in the lower foothills the soil can be sandy loam over heavy sandy loam, or loam over clay loam. Above 2000 feet, it is typically loam over clay loam with cobblestones. An unusual feature of foothills soil is the serpentine outcropping. This combines poor drainage with toxic levels of magnesium. If you need to grow in a serpentine soil area, use raised beds. The serpentine soil under the beds will not provide adequate drainage. Another foothill soil issue that makes for poor drainage is “layered soil”. Soil naturally transitions from one kind to another, but layered soil means soil that changes abruptly, making it hard for water to move through easily. Layered soil occurs naturally (soil on top of rock or a clay pan) and can also be created by digging with rototillers and heavy equipment. Check for poor grading, over-irrigation, and thatched lawns Before you label your soil the culprit, walk your garden and evaluate the grading. It is possible that at the time of your home’s construction, or during a later landscaping project,the soil was graded so the water drained toward an area with no easy outlet. -

Northeast Organic Farming Association of Vermont, Inc
VERMONT ORGANIC FARMERS CERTIFICATION GUIDELINES 2018 Vermont Organic Farmers, LLC (VOF) and the Northeast Organic Farming Association of Vermont, Inc. (NOFA-VT) Vermont Organic Farmers, LLC (VOF) Is the USDA accredited organic certification program of NOFA-VT. NOFA-VT Is a non-profit association of consumers, gardeners and farmers who share a vision of local, organic agriculture. Through education and member participation, NOFA works together to strengthen agriculture in Vermont. Please join us! As a member, you will receive subscriptions to NOFA's regional quarterly publication The Natural Farmer, NOFA-VT’s quarterly newsletter, NOFA Notes, NOFA-VT’s monthly e-news, and you will receive a copy of the Vermont Organic Farm & Food Directory. You will also receive reduced rates at NOFA's annual conference, seasonal workshops, farming supplies and discounts on books and publications. For additional information on membership and NOFA's work, please contact: NOFA-VT · PO Box 697 · Richmond, VT 05477 (802) 434-4122 (NOFA) · (802) 434-3821 (VOF) [email protected] or [email protected] www.nofavt.org 2 Executive Committee Annette Higby, Randolph, VT 728-4955 Brian Norder, Fairfax, VT 849-2000 Sam Smith, Charlotte 985-8018 Review Committee Paul Betz, E Calais, VT 456-8757 Annie Claghorn, Leicester, VT 247-3979 Dave Folino, Bristol, VT 989-5574 Anne Lazor, Westfield, VT 744-6855 Tim Sanford, South Royalton, VT 763-7981 Administration 434-3821 Enid Wonnacott, Executive Director Nicole Dehne, Certification Director Rachel Fussell, Certification Specialist (Crop & Livestock) Winston Rost, Certification Specialist (Processing & Maple) Gregg Stevens, Certification Specialist (Maple, Vegetable & Diverse Operations) Stephanie Walsh, Certification Specialist (Dairy & Livestock) Katy Lash, Certification Program Assistant Laura Nunziata, Quality Assurance Specialist 3 I. -

Phylogenetic Relationships of Rhizoctonia Fungi Within the Cantharellales
fungal biology 120 (2016) 603e619 journal homepage: www.elsevier.com/locate/funbio Phylogenetic relationships of Rhizoctonia fungi within the Cantharellales Dolores GONZALEZa,*, Marianela RODRIGUEZ-CARRESb, Teun BOEKHOUTc, Joost STALPERSc, Eiko E. KURAMAEd, Andreia K. NAKATANIe, Rytas VILGALYSf, Marc A. CUBETAb aInstituto de Ecologıa, A.C., Red de Biodiversidad y Sistematica, Carretera Antigua a Coatepec No. 351, El Haya, 91070 Xalapa, Veracruz, Mexico bDepartment of Plant Pathology, North Carolina State University, Center for Integrated Fungal Research, Campus Box 7251, Raleigh, NC 27695, USA cCBS Fungal Biodiversity Centre, Uppsalalaan 8, 3584 CT Utrecht, The Netherlands dDepartment of Microbial Ecology, Netherlands Institute of Ecology (NIOO/KNAW), Droevendaalsesteeg 10, 6708 PB Wageningen, The Netherlands eUNESP, Faculdade de Ci^encias Agronomicas,^ CP 237, 18603-970 Botucatu, SP, Brazil fDepartment of Biology, Duke University, Durham, NC 27708, USA article info abstract Article history: Phylogenetic relationships of Rhizoctonia fungi within the order Cantharellales were studied Received 2 January 2015 using sequence data from portions of the ribosomal DNA cluster regions ITS-LSU, rpb2, tef1, Received in revised form and atp6 for 50 taxa, and public sequence data from the rpb2 locus for 165 taxa. Data sets 1 January 2016 were analysed individually and combined using Maximum Parsimony, Maximum Likeli- Accepted 19 January 2016 hood, and Bayesian Phylogenetic Inference methods. All analyses supported the mono- Available online 29 January 2016 phyly of the family Ceratobasidiaceae, which comprises the genera Ceratobasidium and Corresponding Editor: Thanatephorus. Multi-locus analysis revealed 10 well-supported monophyletic groups that Joseph W. Spatafora were consistent with previous separation into anastomosis groups based on hyphal fusion criteria.