Group Structure and Dynamics in Black Howlers (Alouatta Pigra): a 7-Year Perspective
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PHYLOGENETIC RELATIONSHIPS AMONG BRAZILIAN HOWLER MONKEYS, GENUS Alouatta (PLATYRRHINI, ATELIDAE), BASED on Γ1-GLOBIN PSEUDOGENE SEQUENCES
Genetics and Molecular Biology, 22, 3, 337-344 Phylogenetic(1999) relationships of Brazilian howler monkeys 337 PHYLOGENETIC RELATIONSHIPS AMONG BRAZILIAN HOWLER MONKEYS, GENUS Alouatta (PLATYRRHINI, ATELIDAE), BASED ON γ1-GLOBIN PSEUDOGENE SEQUENCES Carla Maria Meireles1, John Czelusniak1, Stephen F. Ferrari2, Maria Paula Cruz Schneider2 and Morris Goodman1 ABSTRACT The genus Alouatta (howler monkeys) is the most widely distributed of New World primates, and has been arranged in three species groups: the Central American Alouatta palliata group and the South American Alouatta seniculus and Alouatta caraya groups. While the latter is monotypic, the A. seniculus group encompasses at least three species (A. seniculus, A. belzebul and A. fusca). In the present study, approximately 600 base pairs of the γ1-globin pseudogene were sequenced in the four Brazilian species (A. seniculus, A. belzebul, A. fusca and A. caraya). Maximum parsimony and maximum likelihood methods yielded phylogenetic trees with the same arrangement: {A. caraya [A. seniculus (A. fusca, A. belzebul)]}. The most parsimoni- ous tree had bootstrap values greater than 82% for all groupings, and strength of grouping values of at least 2, supporting the sister clade of A. fusca and A. belzebul. The study also confirmed the presence of a 150-base pair Alu insertion element and a 1.8-kb deletion in the γ1-globin pseudogene in A. fusca, features found previously in the remaining three species. The cladistic classification based on molecular data agrees with those of morphological studies, with the monospecific A. caraya group being clearly differentiated from the A. seniculus group. INTRODUCTION southern Mexico to northern Argentina, and is found in tropical and subtropical forest ecosystems throughout Bra- The systematics of the New World monkeys (infra- zil (Hirsch et al., 1991). -

Conservation of the Caatinga Howler Monkey, Brazil Final Report Brazil
080209 - Conservation of the Caatinga Howler Monkey, Brazil Final Report Brazil, State of Piauí, August 2009 to June 2011. Institutions: Sertões Consultoria Ambiental e Assessoria Overall Aim: Promote the recovery of the Caatinga Howler Monkey through habitat conservation and community involvement Authors: THIERES PINTO AND IGOR JOVENTINO ROBERTO Bill Cartaxo 135, Sapiranga, Fortaleza, Ceará Zip Code: 60833-185 Brazil Emails: [email protected] [email protected] Date: January 23, 2011. Table of Contents SECTION 1……………………………………………………………………………………………………………… pg. 3 SUMMARY …………………………………………………………………………………………..………………… pg. 3 INTRODUCTION ……………………………………………………………………………………………………… pg. 3 PROJECT MEMBERS………………………………………………………………………………………………… pg. 4 SECTION 2. ……………………………………………………………………………………………..……………… pg. 4 AIMS AND OBJECTIVES……………………………………………………………………..……………………… pg. 4 METHODOLOGY……………………………………………………………………………………………………… pg. 4 OUTPUTS AND RESULTS…………………………………………………………………………………………… pg. 5 Goal 1 (A): Determine the interactions between the local communities and the Caatinga Howler Monkey and its habitat…………………………………………………………………………………………………………………… pg. 5 Goal 1 (B.1): Evaluate the perception of the local communities regarding the importance of environmental resources and the conservation of the Caatinga Howler Monkey. …………...………………………………………………… pg. 6 Goal 1 (C): Identify the anthropic threats to the Caatinga Howler Monkey and its habitat conservation. ……… pg. 8 Goal 2: Increase our knowledge on the species ecological requirements (habitat preferences, -

Low Paternity Skew and the Influence of Maternal Kin in an Egalitarian
Low paternity skew and the influence of maternal kin in an egalitarian, patrilocal primate Karen B. Striera,1, Paulo B. Chavesb, Sérgio L. Mendesc, Valéria Fagundesc, and Anthony Di Fioreb,d aDepartment of Anthropology, University of Wisconsin, Madison, WI 53706; bDepartment of Anthropology and Center for the Study of Human Origins, New York University, New York, NY 10003; cDepartamento de Ciências Biológicas, Centro de Ciências Humanas e Naturais, Universidade Federal do Espírito Santo, Maruipe, Vitória, ES 29043-900, Brazil; and dDepartment of Anthropology, University of Texas at Austin, Austin, TX 78712 Contributed by Karen B. Strier, October 12, 2011 (sent for review September 11, 2011) Levels of reproductive skew vary in wild primates living in The fecal samples used for paternity analyses included the 22 multimale groups depending on the degree to which high-ranking infants born between 2005 and 2007 that survived to ≥2.08 y of males monopolize access to females. Still, the factors affecting age, their 21 mothers (representing diverse ages and histories) paternity in egalitarian societies remain unexplored. We combine (Table S1), and the 24 adult males that were possible sires of at unique behavioral, life history, and genetic data to evaluate the least one infant (Table S2). We considered a male to be a po- distribution of paternity in the northern muriqui (Brachyteles tential sire for an infant if he was >5 y and was known to have hypoxanthus), a species known for its affiliative, nonhierarchical completed a copulation before the infant’s estimated conception relationships. We genotyped 67 individuals (22 infants born over a date (birth date minus mean 216.4-d gestation) (16). -

Diets of Howler Monkeys
Chapter 2 Diets of Howler Monkeys Pedro Américo D. Dias and Ariadna Rangel-Negrín Abstract Based on a bibliographical review, we examined the diets of howler mon- keys to compile a comprehensive overview of their food resources and document dietary diversity. Additionally, we analyzed the effects of rainfall, group size, and forest size on dietary variation. Howlers eat nearly all available plant parts in their habitats. Time dedicated to the consumption of different food types varies among species and populations, such that feeding behavior can range from high folivory to high frugivory. Overall, howlers were found to use at least 1,165 plant species, belonging to 479 genera and 111 families as food sources. Similarity in the use of plant taxa as food sources (assessed with the Jaccard index) is higher within than between howler species, although variation in similarity is higher within species. Rainfall patterns, group size, and forest size affect several dimensions of the dietary habits of howlers, such that, for instance, the degree of frugivory increases with increased rainfall and habitat size, but decreases with increasing group size in groups that live in more productive habitats. Moreover, the range of variation in dietary habits correlates positively with variation in rainfall, suggesting that some howler species are habitat generalists and have more variable diets, whereas others are habi- tat specialists and tend to concentrate their diets on certain plant parts. Our results highlight the high degree of dietary fl exibility demonstrated by the genus Alouatta and provide new insights for future research on howler foraging strategies. Resumen Con base en una revisión bibliográfi ca, examinamos las dietas de los monos aulladores para describir exhaustivamente sus recursos alimenticios y la diversidad de su dieta. -
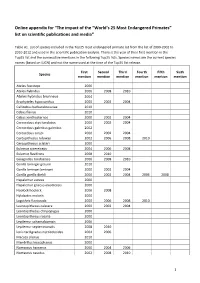
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

BLACK HOWLER MONKEY PRIMATA Family: Cebidae Genus: Alouatta Species: Caraya
BLACK HOWLER MONKEY PRIMATA Family: Cebidae Genus: Alouatta Species: caraya Female with baby Range: eastern Bolivia, northeastern Argentina, Paraguay and southern Brazil Habitat: Various tropical habitats including seasonal (dry) to typical rainforest and wooded savannas Niche: arboreal, diurnal, herbivorous Wild diet: leaves, fruits, other vegetable matter Zoo diet: fruits, vegetables, monkey chow and browse Life Span: Wild 16 – 20, Captivity 23 – 28 years Sexual dimorphism: F 68-75% smaller; M are black while F and young are yellow-brown Location in SF Zoo: Primate Discovery Center APPEARANCE & PHYSICAL ADAPTATIONS: Males are completely black while females and juveniles are yellow-brown or olive-buff. Juvenile males will darken in about 3 months reaching completely black at sexual maturity. The bare underside of the prehensile tail is sensitive to touch and enables the monkey to feel what it is gripping thus assisting when leaping from tree to tree. The hands have a cleft between index finger and middle finger that affords a secure grip since Howlers lack the opposable thumb but have opposable large toes. They usually hold Weight: M - 11-18 lbs objects between their second and third digits on their hand. F - 8 - 12 lbs HBL: 22-36 inches Howlers have enlarged throats, due to an extra-large voice box. The TL: 23-36 inches angle of the lower jaw and the hyoid bone are both greatly enlarged. The special bony pouch (carniculum) is an offshoot of the larynx, just beneath the throat, acts as a resonance box, amplifying the howls. These chambers are larger in the males allowing for the howl to be heard more than a kilometer away in a natural environment. -

World's Most Endangered Primates
Primates in Peril The World’s 25 Most Endangered Primates 2016–2018 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis and Alison Cotton Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2017 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J. and Cotton, A. (eds.). 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. 99 pp. Citation (species): Salmona, J., Patel, E.R., Chikhi, L. and Banks, M.A. 2017. Propithecus perrieri (Lavauden, 1931). In: C. Schwitzer, R.A. Mittermeier, A.B. Rylands, F. Chiozza, E.A. Williamson, E.J. Macfie, J. Wallis and A. Cotton (eds.), Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018, pp. 40-43. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. -

Humboldt, 1812, Primates, Atelidae) in Ecotone Cerrado-Pantanal in the Left Bank of Aquidauana River, Mato Grosso Do Sul, Brazil
Oecologia Australis 16(4): 933-948, Dezembro 2012 http://dx.doi.org/10.4257/oeco.2012.1604.15 DIET AND ACTIVITY PATTERNS OF BLACK HOWLER MONKEYS ALOUATTA CARAYA (HUMBOLDT, 1812, PRIMATES, ATELIDAE) IN ECOTONE CERRADO-PANTANAL IN THE LEFT BANK OF AQUIDAUANA RIVER, MATO GROSSO DO SUL, BRAZIL José Rímoli1, 2,3*, Rodrigo dos Santos Nantes1& Antônio Édson Lázaro Júnior1 1 Universidade Federal de Mato Grosso do Sul (UFMS), Licenciatura em Ciências Biológicas, Campus de Aquidauana, Aquidauana, Mato Grosso do Sul, MS, Brasil, Caixa Postal: 135, Av. Oscar Trindade de Barros 740, CEP: 79.200-000 - Bairro da Serraria. 2 Programa de Pós-Graduação, Mestrado em Biologia Animal, Universidade Federal de Mato Grosso do Sul (UFMS), Campus de Campo Grande, Campo Grande, Mato Grosso do Sul, MS, Brasil. Caixa Postal: 549, Av. Costa e Silva s/n, CEP: 79070-900. 3 Geopark Bodoquena-Pantanal, FUNDECT, Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul, Rua São Paulo, 1436, CEP 79.010-050. E-mail: [email protected], [email protected], [email protected] ABSTRACT The diet and activity patterns of a group of black howler monkeys (Alouatta caraya) were monitored on the left bank of the Aquidauana river over 11 months, from September 2008 to July 2009. The group was composed of eight individuals, two adult males, three females and three immature including subadults and infants. Quantitative data were collected using scan sampling method for 5 minutes with an interval of 15 minutes. The general activities budget (n = 6434 records) was 64.7% rest, 18.5% travel, 10.1% feeding, 4.4% for social behavior and 2.3% for miscellaneous behaviors. -

Vânia Luciane Alves Garcia
Neotropical Primates 13(Suppl.), December 2005 79 SURVEY AND STATUS OF THE MURIQUIS BRACHYTELES ARACHNOIDES IN THE SERRA DOS ÓRGÃOS NATIONAL PARK, RIO DE JANEIRO Vânia Luciane Alves Garcia Seção de Mastozoologia, Departamento de Vertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, e-mail: <[email protected]> Abstract Th e Serra dos Órgãos National Park protects 11,800 ha of Atlantic forest in the state of Rio de Janeiro (22°30'S, 43°06'W; 300 to 2,263 m above sea level). Vegetation types include montane dense evergreeen forest up to altitudes of 1,800 m; cloud forest from 1,800 to 2,000 m; and high altitude grassland above 2,000 m. Th is paper reports on surveys carried out in 10 localities in the park specifi cally to obtain a minimum estimate of the population of northern muriquis (Brachyteles arachnoides). Muriquis were sighted 26 times at altitudes ranging from 800 to 1,500 m. It is possible that they belonged to four groups in which case the minimum number of individuals recorded would be 56. If in fact the sightings were of just two groups, the minimum number of individuals would be 32. Black-horned capuchin (Cebus nigritus) and brown howler mon- keys (Alouatta guariba) were also recorded for the park. Th e buff y-tufted-ear marmoset (Callithrix aurita) was not seen. Key Words – primates, muriqui, Brachyteles, Serra dos Órgãos, Atlantic forest, Brazil Introduction (22°30'S, 43°06'W) (Fig. 1). A number of rivers supplying water to urban centers in the lowlands have their sources in Th ere is now some quite substantial information on the these mountains, including the rios Paquequer, Soberbo, populations of the northern muriqui in Minas Gerais Jacó, Bananal, Bonfi m and Santo Aleixo. -

Ecology of Guatemalan Howler Monkeys (Alouatta Pigra Lawrence)
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1980 Ecology of Guatemalan howler monkeys (Alouatta pigra Lawrence) Janene M. Caywood The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Caywood, Janene M., "Ecology of Guatemalan howler monkeys (Alouatta pigra Lawrence)" (1980). Graduate Student Theses, Dissertations, & Professional Papers. 7254. https://scholarworks.umt.edu/etd/7254 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. COPYRIGHT ACT OF 1976 Th is is an unpublished manuscript in which copyright sub s is t s . Any further r e p r in t in g of it s contents must be approved BY THE AUTHOR. MANSFIELD L ibrary Un iv e r s it y of Montana Da t e : 1 9 g 0 Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. ECOLOGY OF GUATEMALAN HOWLER MONKEYS (AToutta piqra Lawrence) by Janene M. Caywood B.S., Oregon State University, 1976 Presented in partial fu lfillm e n t of the requirements for the degree of Master of Arts UNIVERSITY OF MONTANA 1980 Approved by: Chairman, Board of E^amfners Dean, Graduate SchTTol a Date Reproduced with permission of the copyright owner. -

The Effects of Habitat Disturbance on the Populations of Geoffroy's Spider Monkeys in the Yucatan Peninsula
The Effects of Habitat Disturbance on the Populations of Geoffroy’s Spider Monkeys in the Yucatan Peninsula PhD thesis Denise Spaan Supervisor: Filippo Aureli Co-supervisor: Gabriel Ramos-Fernández August 2017 Instituto de Neuroetología Universidad Veracruzana 1 For the spider monkeys of the Yucatan Peninsula, and all those dedicated to their conservation. 2 Acknowledgements This thesis turned into the biggest project I have ever attempted and it could not have been completed without the invaluable help and support of countless people and organizations. A huge thank you goes out to my supervisors Drs. Filippo Aureli and Gabriel Ramos- Fernández. Thank you for your guidance, friendship and encouragement, I have learnt so much and truly enjoyed this experience. This thesis would not have been possible without you and I am extremely proud of the results. Additionally, I would like to thank Filippo Aureli for all his help in organizing the logistics of field work. Your constant help and dedication to this project has been inspiring, and kept me pushing forward even when it was not always easy to do so, so thank you very much. I would like to thank Dr. Martha Bonilla for offering me an amazing estancia at the INECOL. Your kind words have encouraged and inspired me throughout the past three years, and have especially helped me to get through the last few months. Thank you! A big thank you to Drs. Colleen Schaffner and Jorge Morales Mavil for all your feedback and ideas over the past three years. Colleen, thank you for helping me to feel at home in Mexico and for all your support! I very much look forward to continue working with all of you in the future! I would like to thank the CONACYT for my PhD scholarship and the Instituto de Neuroetología for logistical, administrative and financial support. -

Phylogenetic Analyses Suggest Independent Origins for Trichromatic Color Vision in Apes and Old World Monkeys
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.23.003855; this version posted March 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Phylogenetic analyses suggest independent origins for trichromatic color vision in apes and Old World monkeys Jessica Toloza-Villalobos1 & Juan C. Opazo1 ,2 1Instituto de Ciencias Ambientales y Evolutivas, Facultad de Ciencias, Universidad Austral de Chile, Valdivia, Chile. 2Millennium Nucleus of Ion Channels-associated Diseases (MiNICAD). Corresponding authors: Jessica Toloza-Villalobos ([email protected]) Juan C. Opazo ([email protected]) Phone: +56 63 2221674 Running title: Keywords: trichromatic color vision, opsin gene, gene duplication, primate color vision bioRxiv preprint doi: https://doi.org/10.1101/2020.03.23.003855; this version posted March 23, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract In catarrhine primates, trichromatic color vision is associated with the presence of three opsin genes that absorb light at three different wavelengths. The OPN1LW and OPN1MW genes are found on the X chromosome. Their proximity and similarity suggest that they originated from a duplication event in the catarrhine ancestor. In this study we use the primate genomes available in public databases to study the duplicative history of the OPN1LW and OPN1MW genes and characterize their spectral sensitivity. Our results reveal a phylogenetic tree that shows a clade containing all X-linked opsin paralogs found in Old World monkeys to be related to a clade containing all X-linked opsin paralogs identified in apes, suggesting that routine trichromacy originated independently in apes and Old World monkeys.