Zoonosis Update Lyme Borreliosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vector Hazard Report: Ticks of the Continental United States
Vector Hazard Report: Ticks of the Continental United States Notes, photos and habitat suitability models gathered from The Armed Forces Pest Management Board, VectorMap and The Walter Reed Biosystematics Unit VectorMap Armed Forces Pest Management Board Table of Contents 1. Background 4. Host Densities • Tick-borne diseases - Human Density • Climate of CONUS -Agriculture • Monthly Climate Maps • Tick-borne Disease Prevalence maps 5. References 2. Notes on Medically Important Ticks • Ixodes scapularis • Amblyomma americanum • Dermacentor variabilis • Amblyomma maculatum • Dermacentor andersoni • Ixodes pacificus 3. Habitat Suitability Models: Tick Vectors • Ixodes scapularis • Amblyomma americanum • Ixodes pacificus • Amblyomma maculatum • Dermacentor andersoni • Dermacentor variabilis Background Within the United States there are several tick-borne diseases (TBD) to consider. While most are not fatal, they can be quite debilitating and many have no known treatment or cure. Within the U.S., ticks are most active in the warmer months (April to September) and are most commonly found in forest edges with ample leaf litter, tall grass and shrubs. It is important to check yourself for ticks and tick bites after exposure to such areas. Dogs can also be infected with TBD and may also bring ticks into your home where they may feed on humans and spread disease (CDC, 2014). This report contains a list of common TBD along with background information about the vectors and habitat suitability models displaying predicted geographic distributions. Many tips and other information on preventing TBD are provided by the CDC, AFPMB or USAPHC. Back to Table of Contents Tick-Borne Diseases in the U.S. Lyme Disease Lyme disease is caused by the bacteria Borrelia burgdorferi and the primary vector is Ixodes scapularis or more commonly known as the blacklegged or deer tick. -

Mitochondrial DNA Sequence Variation in Ixodes Paci®Cus (Acari: Ixodidae)
Heredity 83 (1999) 378±386 Received 20 August 1998, accepted 7 July 1999 Mitochondrial DNA sequence variation in Ixodes paci®cus (Acari: Ixodidae) DOUGLAS E. KAIN*, FELIX A. H. SPERLING , HOWELL V. DALY & ROBERT S. LANE Department of Environmental Science, Policy and Management, Division of Insect Biology, University of California at Berkeley, Berkeley, CA 94720, U.S.A. The western black-legged tick, Ixodes paci®cus, is a primary vector of the spirochaete, Borrelia burgdorferi, that causes Lyme disease. We used variation in a 355-bp DNA portion of the mitochondrial cytochrome oxidase III gene to assess the population structure of the tick across its range from British Columbia to southern California and east to Utah. Ixodes paci®cus showed considerable haplotype diversity despite low nucleotide diversity. Maximum parsimony and isolation- by-distance analyses revealed little genetic structure except between a geographically isolated Utah locality and all other localities. Loss of mtDNA polymorphism in Utah ticks is consistent with a post- Pleistocene founder event. The pattern of genetic dierentiation in the continuous part of the range of Ixodes paci®cus reinforces recent recognition of the diculties involved in using genetic frequency data to infer gene ¯ow and migration. Keywords: gene ¯ow, Ixodes paci®cus, Ixodidae, Lyme disease, mitochondrial DNA, population structure. Introduction stages of I. paci®cus each feed once and then drop from the host to moult or lay eggs under leaf litter (Peavey & The western black-legged tick, Ixodes paci®cus, is one of Lane, 1996). Eective gene ¯ow is likely to occur only the most medically important ticks in western North when there is sucient rainfall, ground cover and soil America. -

Blood Smear Analysis in Babesiosis, Ehrlichiosis, Relapsing Fever, Malaria, and Chagas Disease
REVIEW STEVE M. BLEVINS, MD RONALD A. GREENFIELD, MD* MICHAEL S. BRONZE, MD CME Assistant Professor of Medicine, Section Professor of Medicine, Section of Infectious Professor of Medicine, Section of Infectious CREDIT of General Internal Medicine, Department Diseases, Department of Medicine, University Diseases, Chair of Department of Medicine, of Medicine, University of Oklahoma of Oklahoma Health Sciences Center and the University of Oklahoma Health Sciences Center Health Sciences Center, Oklahoma City Oklahoma City Veterans Administration and the Oklahoma City Veterans Administration Medical Center Medical Center Blood smear analysis in babesiosis, ehrlichiosis, relapsing fever, malaria, and Chagas disease ■ ABSTRACT LOOD SMEAR ANALYSIS, while commonly B used to evaluate hematologic condi- Blood smear analysis is especially useful for diagnosing tions, is infrequently used to diagnose infec- five infectious diseases: babesiosis, ehrlichiosis, relapsing tious diseases. This is because of the rarity of fever due to Borrelia infection, malaria, and American diseases for which blood smear analysis is indi- trypanosomiasis (Chagas disease). It should be performed cated. Consequently, such testing is often in patients with persistent or recurring fever or in those overlooked when it is diagnostically impor- who have traveled to the developing world or who have tant. a history of tick exposure, especially if accompanied by Nonspecific changes may include mor- hemolytic anemia, thrombocytopenia, or phologic changes in leukocytes and erythro- 1 hepatosplenomegaly. cytes (eg, toxic granulations, macrocytosis). And with certain pathogens, identifying ■ KEY POINTS organisms in a peripheral blood smear allows for a rapid diagnosis. In the United States, malaria and American This paper discusses the epidemiology, trypanosomiasis principally affect travelers from the clinical manifestations, laboratory findings, developing world. -

Inflammatory Or Infectious Hair Disease? a Case of Scalp Eschar and Neck Lymph Adenopathy After a Tick Bite
Case Report ISSN: 2574 -1241 DOI: 10.26717/BJSTR.2021.35.005688 Adherent Serous Crust of the Scalp: Inflammatory or Infectious Hair Disease? A Case of Scalp Eschar and Neck Lymph Adenopathy after a Tick Bite Starace M1, Vezzoni R*2, Alessandrini A1 and Piraccini BM1 1Dermatology - IRCCS, Policlinico Sant’Orsola, Department of Specialized, Experimental and Diagnostic Medicine, Alma Mater Studiorum, University of Bologna, Italy 2Dermatology Clinic, Maggiore Hospital, University of Trieste, Italy *Corresponding author: Roberta Vezzoni, Dermatology Clinic, Maggiore Hospital, University of Trieste, Italy ARTICLE INFO ABSTRACT Received: Published: April 17, 2021 The appearance of a crust initially suggests inflammatory scalp diseases, although infectious diseases such as impetigo or insect bites should also be considered among April 27, 2021 the differential diagnoses. We report a case of 40-year-old woman presentedB. Burgdorferi to our, Citation: Starace M, Vezzoni R, Hair Disease Outpatient Service with an adherent serous crust on the scalp and lymphadenopathy of the neck. Serological tests confirmed the aetiology of while rickettsia infection was excluded. Lyme borreliosis can mimic rickettsia infection Alessandrini A, Piraccini BM. Adherent and may present as scalp eschar and neck lymphadenopathy after a tick bite (SENLAT). Serous Crust of the Scalp: Inflammatory Appropriate tests should be included in the diagnostic workup of patients with necrotic or Infectious Hair Disease? A Case of Scalp scalpKeywords: eschar in order to promptly set -

Melioidosis in Birds and Burkholderia Pseudomallei Dispersal, Australia
LETTERS 5. Mätz-Rensing K, Jentsch KD, Rensing S, Melioidosis in Birds However, these are mostly birds Langenhuynsen S, Verschoor E, Niphuis in captivity and often exotic to the H, et al. Fatal herpes simplex infection in and Burkholderia a group of common marmosets (Callithrix location, suggesting potential reduced jacchus). Vet Pathol. 2003;40:405–11. pseudomallei immunity. Little is known about doi:10.1354/vp.40-4-405 Dispersal, Australia melioidosis in wild birds. In Sabah, 6. Bruno SF, Liebhold M, Mätz-Rensing K, Malaysia, only 1 of 440 wild birds Romão MA, Didier A, Brandes A, et al. Herpesvirus infection in free-living black- To the Editor: Melioidosis is an admitted to a research center over 9 tufted-ear marmoset (Callithrix penicil- emerging infectious disease of humans years was found to have melioidosis lata E. Geoffroyi 1812) at the state park of and animals caused by the gram- (6). Serra da Tiririca, Niterói, Rio de Janeiro, negative bacterium Burkholderia Although birds are endotherms, Brazil. Berl Munch Tierarztl Wochenschr. 1997;110:427–30. pseudomallei, which inhabits soil and with high metabolic rates and body 7. Kalter SS, Heberling RL. Comparative surface water in the disease-endemic temperature (40°C–43°C) protecting virology of primates. Bacteriol Rev. regions of Southeast Asia and northern them from many diseases, some birds 1971;35:310–64. Australia (1). The aim of this study appear more susceptible to melioidosis. 8. Mansfi eld K. Emerging and re-emerging infectious diseases of nonhuman primates. was to assess the potential for birds Indeed, high body temperature would Proceedings of the American College to spread B. -

Can Leptospirosis Be Treated Without Any Kind of Medication?
ISSN: 2573-9565 Research Article Journal of Clinical Review & Case Reports Can Leptospirosis Be Treated Without Any Kind of Medication? Huang W L* *Corresponding author Huang Wei Ling, Rua Homero Pacheco Alves, 1929, Franca, Sao Paulo, Infectologist, general practitioner, nutrition doctor, acupuncturist, 14400-010, Brazil, Tel: (+55 16) 3721-2437; E-mail: [email protected] pain management, Medical Acupuncture and Pain Management Clinic, Franca, Sao Paulo, Brazil Submitted: 16 Apr 2018; Accepted: 23 Apr 2018; Published: 10 May 2018 Abstract Introduction: Leptospirosis is an acute infectious disease caused by pathogenic Leptospira. Spread in a variety of ways, though the digestive tract infection is the main route of infection. As the disease pathogen final position in the kidney, the urine has an important role in the proliferation of the disease spreading [1]. Purpose: The purpose of this study was to show if leptospirosis can be treated without any kind of medication. The methodology used was the presentation of one case report of a woman presenting three days of generalized pain all over her body, especially in her muscles, mainly the calves of her legs, fever, headache and trembling. A blood exam was asked, as well as serology and acupuncture to relieve her symptoms. Findings: she recovered very well after five sessions of Acupuncture once a day. A month later, she came back with the results of her serology: it was positive leptospirosis. Conclusion: In this case, leptospirosis was cured without the use any kind of medication, being acupuncture a good therapeutic option, reducing the necessity of the patient’s admittance into a hospital, minimizing the costs of the treatmentand restoring the patient to a normal life very quickly. -

Anaplasmosis: an Emerging Tick-Borne Disease of Importance in Canada
IDCases 14 (2018) xxx–xxx Contents lists available at ScienceDirect IDCases journal homepage: www.elsevier.com/locate/idcr Case report Anaplasmosis: An emerging tick-borne disease of importance in Canada a, b,c d,e e,f Kelsey Uminski *, Kamran Kadkhoda , Brett L. Houston , Alison Lopez , g,h i c c Lauren J. MacKenzie , Robbin Lindsay , Andrew Walkty , John Embil , d,e Ryan Zarychanski a Rady Faculty of Health Sciences, Max Rady College of Medicine, Department of Internal Medicine, University of Manitoba, Winnipeg, MB, Canada b Cadham Provincial Laboratory, Government of Manitoba, Winnipeg, MB, Canada c Rady Faculty of Health Sciences, Max Rady College of Medicine, Department of Medical Microbiology and Infectious Diseases, University of Manitoba, Winnipeg, MB, Canada d Rady Faculty of Health Sciences, Max Rady College of Medicine, Department of Internal Medicine, Section of Medical Oncology and Hematology, University of Manitoba, Winnipeg, MB, Canada e CancerCare Manitoba, Department of Medical Oncology and Hematology, Winnipeg, MB, Canada f Rady Faculty of Health Sciences, Max Rady College of Medicine, Department of Pediatrics and Child Health, Section of Infectious Diseases, Winnipeg, MB, Canada g Rady Faculty of Health Sciences, Max Rady College of Medicine, Department of Internal Medicine, Section of Infectious Diseases, University of Manitoba, Winnipeg, MB, Canada h Rady Faculty of Health Sciences, Max Rady College of Medicine, Department of Community Health Sciences, University of Manitoba, Winnipeg, MB, Canada i Public Health Agency of Canada, National Microbiology Laboratory, Zoonotic Diseases and Special Pathogens, Winnipeg, MB, Canada A R T I C L E I N F O A B S T R A C T Article history: Human Granulocytic Anaplasmosis (HGA) is an infection caused by the intracellular bacterium Received 11 September 2018 Anaplasma phagocytophilum. -

Tick-Borne Relapsing Fever CLAY ROSCOE, M.D., and TED EPPERLY, M.D., Family Medicine Residency of Idaho, Boise, Idaho
Tick-Borne Relapsing Fever CLAY ROSCOE, M.D., and TED EPPERLY, M.D., Family Medicine Residency of Idaho, Boise, Idaho Tick-borne relapsing fever is characterized by recurring fevers separated by afebrile periods and is accompanied by nonspecific constitutional symptoms. It occurs after a patient has been bitten by a tick infected with a Borrelia spirochete. The diagnosis of tick-borne relapsing fever requires an accurate characterization of the fever and a thorough medical, social, and travel history of the patient. Findings on physical examination are variable; abdominal pain, vomiting, and altered sensorium are the most common symptoms. Laboratory confirmation of tick-borne relapsing fever is made by detection of spirochetes in thin or thick blood smears obtained during a febrile episode. Treatment with a tetracycline or macrolide antibiotic is effective, and antibiotic resistance is rare. Patients treated for tick-borne relapsing fever should be monitored closely for Jarisch- Herxheimer reactions. Fatalities from tick-borne relapsing fever are rare in treated patients, as are subsequent Jarisch-Herxheimer reactions. Persons in endemic regions should avoid rodent- and tick-infested areas and use insect repellents and protective clothing to prevent tick bites. (Am Fam Physician 2005;72:2039-44, 2046. Copyright © 2005 American Academy of Family Physicians.) S Patient information: ick-borne relapsing fever (TBRF) develop with TBRF, with long-term sequelae A handout on tick-borne is transmitted by Ornithodoros that may be permanent. Reviewing a broad relapsing fever, written by 1,3-6 the authors of this article, ticks infected with one of sev- differential diagnosis (Table 1 ) for fever is provided on page 2046. -

Lyme Disease and Tick-Borne Infections User Manual
SCOTTISH MICROBIOLOGY REFERENCE LABORATORY, INVERNESS: LYME DISEASE AND TICK-BORNE INFECTIONS USER MANUAL 1 Amended 23 September 2020 CONTENTS Section Page 1 Introduction 3 2 Contact details and key personnel 3 3 Opening hours 4 4 Service provided 4 4.1 Samples and turnaround times 4 4.2 Laboratory tests 5 4.3 Specialist advice 5 5 Clinical Information 6 6 Referral criteria 6 7 Specimen and request form labelling 7 8 Specimen transportation 8 9 Charges 8 10 Results 8 11 Treatment 8 12 Prevention 8 13 SLDTRL request form 8 (Form MF023) 14 SLDTRL developments 9 15 References 9 16 Laboratory diagnosis of Lyme borreliosis algorithm Appendix 2 Amended 23 September 2020 1.0 Introduction The newly established Scottish Lyme Disease and Tick-borne Infections Reference Laboratory (SLDTRL) is provided by NHS Highland at Raigmore Hospital, Inverness. The aim of SLDTRL is to provide more comprehensive and standardised testing for Lyme disease and other tick-borne infections and to improve the epidemiological data provided to Health Protection Scotland (HPS). Lyme disease is caused by bacteria from the Borrelia burgdorferi sensu lato complex. In the UK the bacteria is transmitted to humans through the bite of infected, hard bodied, Ixodes ricinus ticks. Borrelia miyamotoi disease, which presents as a relapsing fever, can also be transmitted by Ixodes ricinus ticks. It is an emerging disease caused by B. miyamotoi bacteria, which are from the relapsing fever group of borrelia, genetically distinct from those that cause Lyme disease. Human granulocytic anaplasmosis (HGA), also an acute febrile illness transmitted by Ixodid ticks, is an infection caused by the bacterium Anaplasma phagocytophilum. -

Leptospirosis Associated Equine Recurrent Uveitis Answers to Your Important Questions What Is Leptospirosis Associated Equine Recurrent Uveitis (LAERU)?
Lisa Dauten, DVM Tri-State Veterinary Services LLC " Leptospirosis Associated Equine Recurrent Uveitis Answers to your Important Questions! What is Leptospirosis Associated Equine Recurrent Uveitis (LAERU)? Let’s start by breaking down some terminology.! Uveitis- inflammation of the uvea. Resulting in cloudiness of the eye, pain, and potential blindness. Also know as “Moon Blindness”. Caused by trauma, infection, or corneal disease.! Uvea- part of the eye containing the iris, ciliary body, and choroid. It keeps the lens of the eye in place, maintains fluid in the eye, and keeps things in the blood from entering the inside of the eye (blood-ocular barrier). ! Recurrent Uveitis- inflammation of the uvea that sporadically reoccurs through out a horses life time. Each time there is a reoccurring episode, the damage to the eye is made worse, eventually leading to permanent damage and potential blindness. ! Leptospirosis- bacteria found in the environment shed in the urine of wildlife and livestock. Horses usually are exposed when grazing pastures or drinking from natural water sources.! LAERU- Recurrent Uveitis in horses caused by Leptospirosis.! What are the clinical signs of Uveitis? Uveitis can come on very suddenly. A lot of times horses present with severe pain in the eye, tearing, squinting, and rubbing face. The eye itself is cloudy, white or blue in color. Sometimes the signs are not as dramatic. The color change of the eye may progress slowly. In these cases, horse owners may mistake the changes for cataracts.! What do I do if I think my horse has Uveitis? Call your veterinarian to request an appointment. -

Erythema Marginatum
Figurative Erythemas Michelle Goedken, DO Affiliated Dermatology Scottsdale, AZ Figurative Erythemas • Erythema annulare centrifugum • Erythema marginatum • Erythema migrans • Erythema gyratum repens • Erythema multiforme Erythemas • Erythemas represent a change in the color of the skin that is due to the dilation of blood vessels, especially those in the papillary and reticular dermis • The color is blanchable and most last for days to months • Figurative erythemas have an annular, arciform or polycyclic appearance ERYTHEMA ANNULARE CENTRIFUGUM ERYTHEMA ANNULARE CENTRIFUGUM • Pathogenesis: EAC represents a reaction pattern or hypersensitivity to one of many antigens – IL-2 and TNF-alpha may have a role – Most patients do not have an underlying disease identified ERYTHEMA ANNULARE CENTRIFUGUM • Associated with: – Infection » Dermatophytes and other fungi (Candida and Penicillium in blue cheese) » Viruses: poxvirus, EBV, VZV, HIV » Parasites and ectoparasites – Drugs: diuretics, antimalarials, gold, NSAIDs, finasteride, amitriptyline, etizolam, Ustekinumab (2012) ERYTHEMA ANNULARE CENTRIFUGUM – Foods – Autoimmune endocrinopathies – Neoplasms (lymphomas and leukemias) – Pregnancy – Hypereosinophilic syndrome – Lupus (2014) ERYTHEMA ANNULARE CENTRIFUGUM http://www.dermaamin.com Rongioletti, F., Fausti, V., & Parodi, A ERYTHEMA ANNULARE CENTRIFUGUM • 2 major forms: – Superficial: classic trailing scale, may have associated pruritus – Deep: infiltrated borders, usually no scale, edges are elevated, usually not pruritic ERYTHEMA ANNULARE CENTRIFUGUM -
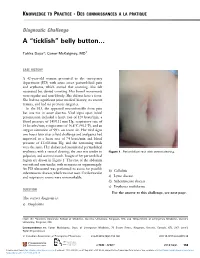
Belly Button…
KNOWLEDGE TO PRACTICE DES CONNAISSANCES À LA PRATIQUE Diagnostic Challenge A “ticklish” belly button… Tahira Daya*; Conor McKaigney, MD† CASE HISTORY A 42-year-old woman presented to the emergency department (ED) with acute onset periumbilical pain and erythema, which started that morning. She felt nauseated but denied vomiting. Her bowel movements were regular and non-bloody. She did not have a fever. She had no significant prior medical history, no recent trauma, and had no previous surgeries. In the ED, she appeared uncomfortable from pain but was not in acute distress. Vital signs upon initial presentation included a heart rate of 120 beats/min, a blood pressure of 145/111 mm Hg, respiratory rate of 16 breaths/min, temperature of 36.8°C (98.2°F), and an oxygen saturation of 99% on room air. Her vital signs two hours later after a fluid challenge and analgesics had improved to a heart rate of 74 beats/min and blood pressure of 132/83 mm Hg, and the remaining vitals were the same. Her abdomen demonstrated periumbilical erythema, with a central clearing; the area was tender to Figure 1. Periumbilical rash with central clearing. palpation, and warm to touch. Images of her periumbilical region are shown in Figure 1. The rest of the abdomen was soft and non-tender, with no masses or organomegaly. An ED ultrasound was performed to assess for possible b) Cellulitis subcutaneous abscess, which was not seen. Cardiovascular and respiratory exams were unremarkable. c) Lyme disease d) Subcutaneous abscess e) Erythema multiforme QUESTION For the answer to this challenge, see next page.