Neonatal Hypoxia Ischaemia: Mechanisms, Models, and Therapeutic Challenges
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ryerson University Spring Graduates
Ryerson University Spring Graduates June 2020 Faculty of Arts 2 Faculty of Communication & Design 11 Faculty of Community Services 21 Faculty of Engineering and Architectural Science 35 Faculty of Science 46 Ted Rogers School of Management 54 Yeates School of Graduate Studies 71 The G. Raymond Chang School of Continuing Education 73 Faculty of Arts Pamela Sugiman Dean Faculty of Arts Janice Fukakusa Chancellor Mohamed Lachemi President and Vice-Chancellor Charmaine Hack Registrar Ryerson Gold Medal Presented to Mayah Obadia Geographic Analysis 2 Faculty of Arts Undergraduate Degree Programs Arts and Contemporary Studies Bachelor of Arts (Honours) *Diana Abo Harmouch Carmen Jajjo *Megumi Noteboom *Sima Rebecca Abrams Leya Jasat Valentina Padure Qeyam Amiri Sophie Johnson *Naiomi Marcia Perera Brodie Barrick Babina Kamalanathan Charlotte Jane Prokopec Rebecca Claire Chen Caroline Susan Kewley Regan Reynolds Erin Tanya Clarke Jessica Laurenza Joshua Ricci *Megan Lisa Devoe Claire Lowenstein Kaitlin Anganie Seepersaud *Manpreet Kaur Dhaliwal *Avigayil Margolis Gabriela Skwarko Tatum Lynn Donovan Sara McArthur Julia Macey Sullivan Faith Raha Giahi *Nadia Celeste McNairn *Helen Gillian Webb Meagan Gove *Mahbod Mehrvarz *Michael Worbanski Salem Habtom Andrew Moon Smyrna Wright *William Hanchar *Liana Gabriella Mortin Calum Jacques Potoula Mozas Criminology Bachelor of Arts (Honours) *Annabelle Adjei *Jenna Anne Giannini Veronica Hiu Lam Lee Stanislav Babinets Albina Glatman Karishma Catherine Lutchman Hela Bakhtari Farah Khaled Gregni Simbiat -

Climate Drives Fire Synchrony but Local Factors Control Fire Regime Change In
Climate drives fire synchrony but local factors control fire regime change in northern Mexico 1, 1 2 3 LARISSA L. YOCOM KENT, PETER Z. FULE, PETER M. BROWN, JULIAN CERANO-PAREDES, 4 ~ 1,5 6 7,8 ELADIO CORNEJO-OVIEDO, CITLALI CORTES MONTANO, STACY A. DRURY, DONALD A. FALK, 9 10 11 12 3 JED MEUNIER, HELEN M. POULOS, CARL N. SKINNER, SCOTT L. STEPHENS, AND JOSE VILLANUEVA-DIAZ 1School of Forestry, Northern Arizona University, P.O. Box 15018, Flagstaff, Arizona 86011 USA 2Rocky Mountain Tree-Ring Research, 2901 Moore Lane, Fort Collins, Colorado 80526 USA 3National Institute of Forest, Agriculture, and Livestock Research, National Center of Disciplinary Research on Water, Soil, Plants, and Atmosphere, Km. 6.5 Margen Derecha del Canal Sacramento, C.P. 35140 Gomez Palacio, Durango, Mexico 4Departamento Forestal, Universidad Autonoma Agraria Antonio Narro, Calzada Antonio Narro #1923, Buenavista, C.P. 25315 Saltillo, Coahuila, Mexico 5Instituto de Silvicultura e Industria de la Madera, Universidad Juarez del Estado de Durango, Boulevard del Guadiana #501, Ciudad Universitaria, Torre de Investigacion, C. P. 34120 Durango, Mexico 6USDA Forest Service Pacific Southwest Research Station, 1731 Research Park Drive, Davis, California 95618 USA 7School of Natural Resources and the Environment, University of Arizona, 1064 Lowell Street, Tucson, Arizona 85721 USA 8Laboratory of Tree-Ring Research, University of Arizona, 1215 E. Lowell Street, Tucson, Arizona 85721 USA 9Wisconsin Department of Natural Resources, Science Operations Center, 2801 Progress Road, Madison, Wisconsin 53716 USA 10College of the Environment, Wesleyan University, 284 High Street, Middletown, Connecticut 06459 USA 11USDA Forest Service, Pacific Southwest Research Station, 3644 Avtech Parkway, Redding, California 96002 USA 12Department of Environmental Science, Policy, and Management, University of California, 130 Mulford Hall, Berkeley, Berkeley, California 94720 USA Citation: Yocom Kent, L. -

Inverness Gaelic Society
Inverness Gaelic Society Collection Last Updated Jan 2020 Title Author Call Number Burt's letters from the north of Scotland : with facsimiles of the original engravings (Burt, Edward), d. 1755 941.2 An English Irish dictionary, intended for the use of schools : containing upwards of eight thousand(Connellan, English Thaddeus),words, with d. their 1854 corresponding explanation491.623 in Irish The martial achievements of the Scots nation : being an account of the lives, characters and memorableAbercromby, actions, Patrick of such Scotsmen as have signaliz'd941.1 themselves by the sword at home and abroad and a survey of the military transactions wherein Scotland or Scotsmen have been remarkably concern'd from thefirst establishment of the Scots monarchy to this present time Officers and graduates of University & King's College, Aberdeen MVD-MDCCCLX Aberdeen. University and King's College 378.41235 The Welsh language 1961-1981 : an interpretative atlas Aitchison, J. W. 491.66 Scottish fiddlers and their music Alburger, Mary Anne 787.109411 Place-names of Aberdeenshire Alexander, William M. 929.4 Burn on the hill : the story of the first 'Compleat Munroist' Allan, Elizabeth B.BUR The Bridal Caölchairn; and other poems Allan, John Carter, afetrwards Allan (John Hay) calling808.81 himself John Sobiestki Stolberg Stuart Earail dhurachdach do pheacaich neo-iompaichte Alleine, Joseph 234.5 Earail Dhurachdach do pheacaich neo-iompaichte Alleine, Joseph 234.5 Leabhar-pocaid an naoimh : no guth Dhe anns na Geallaibh Alleine, Joseph 248.4 My little town of Cromarty : the history of a northern Scottish town Alston, David, 1952- 941.156 An Chomhdhail Cheilteach Inbhir Nis 1993 : The Celtic Congress Inverness 1993 An Chomhdhail Cheilteach (1993 : Scotland) 891.63 Orain-aon-neach : Leabhar XXI. -
Bdo International Directory 2017
International Directory 2017 Latest version updated 5 July 2017 1 ABOUT BDO BDO is an international network of public accounting, tax and advisory firms, the BDO Member Firms, which perform professional services under the name of BDO. Each BDO Member Firm is a member of BDO International Limited, a UK company limited by guarantee. The BDO network is governed by the Council, the Global Board and the Executive (or Global Leadership Team) of BDO International Limited. Service provision within the BDO network is coordinated by Brussels Worldwide Services BVBA, a limited liability company incorporated in Belgium with VAT/BTW number BE 0820.820.829, RPR Brussels. BDO International Limited and Brussels Worldwide Services BVBA do not provide any professional services to clients. This is the sole preserve of the BDO Member Firms. Each of BDO International Limited, Brussels Worldwide Services BVBA and the member firms of the BDO network is a separate legal entity and has no liability for another such entity’s acts or omissions. Nothing in the arrangements or rules of BDO shall constitute or imply an agency relationship or a partnership between BDO International Limited, Brussels Worldwide Services BVBA and/or the member firms of the BDO network. BDO is the brand name for the BDO network and all BDO Member Firms. BDO is a registered trademark of Stichting BDO. © 2017 Brussels Worldwide Services BVBA 2 2016* World wide fee Income (millions) EUR 6,844 USD 7,601 Number of countries 158 Number of offices 1,401 Partners 5,736 Professional staff 52,486 Administrative staff 9,509 Total staff 67,731 Web site: www.bdointernational.com (provides links to BDO Member Firm web sites world wide) * Figures as per 30 September 2016 including exclusive alliances of BDO Member Firms. -

Annual Report 2009 Annual Report 2009
King’s College, Cambridge Annual Report 2009 Annual Report 2009 Contents The Provost 2 The Fellowship 7 Undergraduates at King’s 18 Graduates at King’s 24 Tutorial 26 Research 32 Library 35 Chapel 38 Choir 41 Staff 43 Development 46 Members’ Information Form 51 Obituaries 55 Information for Members 259 The first and most obvious to the blinking, exploring, eye is buildings. If The Provost you go to the far side of the Market Place and look back, you now see three tall buildings: Great St Mary’s, King’s Chapel, and a taller Market Hostel. First spiky scaffolding reached above the original roof. Now it has all been shrouded in polythene, like a mystery shop window offering. It’ll stay 2 Things could only get better. You wrapped for a year until the major refit is completed next summer. 3 may remember that when I wrote THE PROVOST these notes last year, I had just Moving back into the main college in my exploratory perambulation, I find discovered that the College had more scaffolding. It’s on the Wilkins Screen. It’s on the Chapel, where through written to the local authorities THE PROVOST the summer we’ve moved down the entire South side, cleaning and treating saying that they were unaware of the glazing bars so that they no longer rust, expand, and prise off bits of the my place of residence. The College stone. Face lifts for the fountain and founder’s statue. So I see much activity, did not know where I was and I expensive activity. -
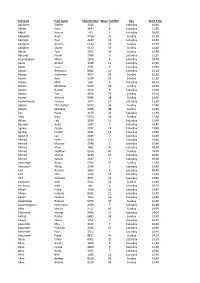
Surname First Name Chip Number Wave Number Day Start Time
Surname First name Chip Number Wave Number Day Start Time Aardoom Sophie 2345 7 Saturday 10:40 Abbott Ciara 2643 8 Saturday 11:00 Abbott Joanne 501 1 Saturday 09:00 Abdulakh Anya 4754 29 Sunday 10:30 Aberson Paul 4232 15 Saturday 13:20 Abington Jeremy 6122 36 Sunday 12:40 Abington Stuart 6123 36 Sunday 12:40 Abram Tom 5971 36 Sunday 12:40 Abuzour Fasial 2938 9 Saturday 11:20 Acquistapace Mario 1876 4 Saturday 09:40 Adam Alistair 3539 12 Saturday 12:20 Adam Lizzy 2731 8 Saturday 11:00 Adams Benjamin 8001 20 Saturday 15:10 Adams Catherine 4917 29 Sunday 10:30 Adams John 5528 32 Sunday 11:20 Adams Matt 1501 3 Saturday 09:20 Adams Matthew 5972 36 Sunday 12:40 Adams Rachel 2346 8 Saturday 11:00 Adams Tim 4870 29 Sunday 10:30 Adams Victoria 6485 38 Sunday 13:20 Adamthwaite Fuchsia 3077 10 Saturday 11:40 Adcock Christopher 5973 36 Sunday 12:40 Adcock Michelle 6365 38 Sunday 13:20 Ade Paula 3119 10 Saturday 11:40 Adey Gary 5974 36 Sunday 12:40 Adlam Lee 3299 11 Saturday 12:00 Agarwal Ankur 2347 7 Saturday 10:40 Agnew Caspar 3932 14 Saturday 13:00 Agnew Hector 3931 14 Saturday 13:00 Agostini Luc 2439 7 Saturday 10:40 Ahmad Helen 4244 17 Saturday 14:00 Ahmad Muaaze 2348 7 Saturday 10:40 Ahmed Irfan 1891 4 Saturday 09:40 Ahmed Quddous 8516 40 Sunday 14:00 Ahmed Samah 4245 17 Saturday 14:00 Ahmed Sohaib 2437 7 Saturday 10:40 Ainscough Andy 5302 31 Sunday 11:00 Ainsworth Philip 2349 7 Saturday 10:40 Aird Alastair 1863 4 Saturday 09:40 Aird Chris 3972 14 Saturday 13:00 Aird Nicholas 3971 14 Saturday 13:00 Aitchison Kate 5312 31 Sunday 11:00 Aitchison -
Affidavit of Mailing with Attachments- July 8, 2019
Exhibit A GLENN GROUNDWATER AUTHORITY NOTICE OF HEARING ON PROPOSED FEE In compliance with California State Law, notice is hereby given that the Glenn Groundwater Authority (GGA) will conduct a public hearing on: July 8, 2019 at 5:30 p.m. at the City of Willows Council Chamber, 201 N. Lassen St., Willows, CA to consider the adoption of a new property related fee in the amount of $1.93 per acre annually for the 2019 Fiscal Year and the subsequent four fiscal years, to fund operations of the GGA. Notificación en Español: Para leer este aviso en Español, visite el sitio web de GGA https://www.countyofglenn.net/dept/agriculture/water- resources/glenn-groundwater-authority o llame (916) 918-2020 para solicitar que se le envíe un aviso por correo o correo electrónico. Background: The GGA is a nine-member, multi-agency Joint Powers Authority that was formed on June 20, 2017. The GGA is the Groundwater Sustainability Agency (GSA) responsible for implementation and compliance of the Sustainable Groundwater Management Act (SGMA) in the Glenn County portion of the Colusa Subbasin (5-21.52). The Board of the GGA is composed of representatives of the following: County of Glenn, City of Orland, City of Willows, Glenn-Colusa Irrigation District, Glide Water District, Princeton-Codora-Glenn/Provident Irrigation District (1 seat), Orland-Artois Water District, and Kanawha Water District. As a groundwater regulating agency, the GGA (in partnership with other adjacent GSAs such as the Colusa Groundwater Authority [CGA]) is tasked with achieving and maintaining sustainable groundwater conditions in the Colusa Subbasin (Basin). -
Traitement Position/Poste Taxable Benefits
Taxable Surname/Nom de Given Name/ Salary Paid/ Benefits/ Employer/Employeur famille Prénom Position/Poste Traitement Avant. impos. City of Oshawa SYMONS-MILROY CINDY Director, Economic Development Services$147,536.22 $660.50 City of Oshawa THURSTON GLENN Manager, Human Resource Services and Safety$108,684.19 $486.64 City of Oshawa THWAITES RODERICK Captain $108,428.43 $426.41 City of Oshawa TOOR MANMOHAN Manager, Construction Services $108,684.48 $486.64 City of Oshawa TUTTON CHRISTOPHER Captain $108,428.43 $426.41 City of Oshawa VINCENT GORDON Captain $102,781.12 $426.41 City of Oshawa WATSON GREGORY Captain $116,514.03 $426.41 City of Oshawa WEBSTER JOHN Firefighter $100,358.86 $370.62 City of Oshawa WHETHAM KENNETH Firefighter $104,162.21 $370.62 City of Oshawa WILSON NANCI Fire Prevention Officer $100,895.00 $426.41 City of Oshawa WILTON KEVIN Firefighter $106,196.34 $370.62 City of Oshawa WOOD GLEN Firefighter $116,762.41 $370.62 City of Oshawa WOOD SCOTT Training Officer $100,295.00 $426.41 City of Oshawa WYLIE J. NICOLE Director, Finance/Treasurer $133,328.26 $598.88 City of Ottawa ADAMS MICHAEL Garage Attendant-Starter Placer 1 $101,457.29 $1,294.89 City of Ottawa AGOCS IMRE Working Supervisor-Mechanic Inspector 1$100,308.87 $1,925.05 City of Ottawa AKESON JEFF Zone Supervisor $134,721.51 $916.32 City of Ottawa ALADAS MOTAZ Section Manager, Systems Communications & Video$117,918.87 $388.32 City of Ottawa ALBERT MARC Fire Prevention Officer $121,776.46 $1,470.50 City of Ottawa ALLAIRE LISA Chief, Corporate Communications $143,738.94 -
Index to 1920-1924 Obituaries in the Canton Repository
Index to 1920-1924 Obituaries in the Canton Repository Published by the Stark County District Library of Canton, Ohio 2005 Index to 1920-1924 Obituaries in the Canton Repository The following is an alphabetical listing, by the deceased’s surname, of obituaries that appeared in the Canton, Ohio newspaper, The Repository. It was compiled by the library staff during the course of the year in the hope that it would be a useful tool to genealogy, as well as other researchers. To use this index, simply locate the name of the person whose obituary you wish to find. The entries will appear as follows: Miller William A. (Estella M.) Mrs. 1924 Jan. 13 30 The first column is the name of the deceased. The second gives the date on which the item appeared. And the third tells the page number containing the obituary. You may find one name with multiple listings showing different dates and locations in the paper. This indicates consecutive listings for that individual. I encourage you to view each for additional information. Surname Given Name Maiden Title Year Mth. Day Pg. ?amian Dan 1923 Aug. 9 7 Abas Pasha 1921 June 13 1 Abbenante Vincenza Mrs. 1921 Feb. 5 2 Abeggien Abram 1921 Mar. 31 5 Abel Albert 1921 Aug. 6 2 Abell Catherine Mrs. 1921 Jan. 11 6 Abell Catherine Mrs. 1921 Jan. 12 8 Abraham Joseph 1924 April 8 18 Abraham Joseph 1924 April 9 18 Abrigg Lawrence 1923 July 9 1 Ackelson James 1924 May 12 10 Acker Charles 1920 Mar. 13 4 Acker Jacob S. -

LCSH Section L
L (The sound) L1 algebras La Bonte Creek (Wyo.) [P235.5] UF Algebras, L1 UF LaBonte Creek (Wyo.) BT Consonants BT Harmonic analysis BT Rivers—Wyoming Phonetics Locally compact groups La Bonte Station (Wyo.) L.17 (Transport plane) L2TP (Computer network protocol) UF Camp Marshall (Wyo.) USE Scylla (Transport plane) [TK5105.572] Labonte Station (Wyo.) L-29 (Training plane) UF Layer 2 Tunneling Protocol (Computer network BT Pony express stations—Wyoming USE Delfin (Training plane) protocol) Stagecoach stations—Wyoming L-98 (Whale) BT Computer network protocols La Borde Site (France) USE Luna (Whale) L98 (Whale) USE Borde Site (France) L. A. Franco (Fictitious character) USE Luna (Whale) La Bourdonnaye family (Not Subd Geog) USE Franco, L. A. (Fictitious character) LA 1 (La.) La Braña Region (Spain) L.A.K. Reservoir (Wyo.) USE Louisiana Highway 1 (La.) USE Braña Region (Spain) USE LAK Reservoir (Wyo.) La-5 (Fighter plane) La Branche, Bayou (La.) L.A. Noire (Game) USE Lavochkin La-5 (Fighter plane) UF Bayou La Branche (La.) UF Los Angeles Noire (Game) La-7 (Fighter plane) Bayou Labranche (La.) BT Video games USE Lavochkin La-7 (Fighter plane) Labranche, Bayou (La.) L.C.C. (Life cycle costing) La Albarrada, Battle of, Chile, 1631 BT Bayous—Louisiana USE Life cycle costing USE Albarrada, Battle of, Chile, 1631 La Brea Avenue (Los Angeles, Calif.) L.C. Smith shotgun (Not Subd Geog) La Albufereta de Alicante Site (Spain) This heading is not valid for use as a geographic UF Smith shotgun USE Albufereta de Alicante Site (Spain) subdivision. BT Shotguns La Alcarria Plateau (Spain) UF Brea Avenue (Los Angeles, Calif.) L Class (Destroyers : 1939-1948) (Not Subd Geog) USE Alcarria Plateau (Spain) BT Streets—California UF Laforey Class (Destroyers : 1939-1948) La Alcudia Site (Spain) La Brea Pits (Calif.) BT Destroyers (Warships) USE Alcudia Site (Spain) UF La Brea Tar Pits (Calif.) L.