Section 28-4: the Pauli Exclusion Principle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Atomic Orbitals
Visualization of Atomic Orbitals Source: Robert R. Gotwals, Jr., Department of Chemistry, North Carolina School of Science and Mathematics, Durham, NC, 27705. Email: [email protected]. Copyright ©2007, all rights reserved, permission to use for non-commercial educational purposes is granted. Introductory Readings Where are the electrons in an atom? There are a number of Models - a description of models that look to describe the location and behavior of some physical object or electrons in atoms. It is important to know this information, as behavior in nature. Models it helps chemists to make statements and predictions about are often mathematical, describing the behavior of how atoms will react with other atoms to form molecules. The some event. model one chooses will oftentimes influence the types of statements one can make about the behavior – that is, the reactivity – of an atom or group of atoms. Bohr model - states that no One model, the Bohr model, describes electrons as small two electrons in an atom can billiard balls, rotating around the positively charged nucleus, have the same set of four much in the way that planets quantum numbers. orbit around the Sun. The placement and motion of electrons are found in fixed and quantifiable levels called orbits, Orbits- where electrons are or, in older terminology, shells. believed to be located in the There are specific numbers of Bohr model. Orbits have a electrons that can be found in fixed amount of energy, and are identified with the each orbit – 2 for the first orbit, principle quantum number 8 for the second, and so forth. -

Lecture #2: August 25, 2020 Goal Is to Define Electrons in Atoms
Lecture #2: August 25, 2020 Goal is to define electrons in atoms • Bohr Atom and Principal Energy Levels from “orbits”; Balance of electrostatic attraction and centripetal force: classical mechanics • Inability to account for emission lines => particle/wave description of atom and application of wave mechanics • Solutions of Schrodinger’s equation, Hψ = Eψ Required boundaries => quantum numbers (and the Pauli Exclusion Principle) • Electron configurations. C: 1s2 2s2 2p2 or [He]2s2 2p2 Na: 1s2 2s2 2p6 3s1 or [Ne] 3s1 => Na+: [Ne] Cl: 1s2 2s2 2p6 3s23p5 or [Ne]3s23p5 => Cl-: [Ne]3s23p6 or [Ar] What you already know: Quantum Numbers: n, l, ml , ms n is the principal quantum number, indicates the size of the orbital, has all positive integer values of 1 to ∞(infinity) (Bohr’s discrete orbits) l (angular momentum) orbital 0s l is the angular momentum quantum number, 1p represents the shape of the orbital, has integer values of (n – 1) to 0 2d 3f ml is the magnetic quantum number, represents the spatial direction of the orbital, can have integer values of -l to 0 to l Other terms: electron configuration, noble gas configuration, valence shell ms is the spin quantum number, has little physical meaning, can have values of either +1/2 or -1/2 Pauli Exclusion principle: no two electrons can have all four of the same quantum numbers in the same atom (Every electron has a unique set.) Hund’s Rule: when electrons are placed in a set of degenerate orbitals, the ground state has as many electrons as possible in different orbitals, and with parallel spin. -

The Quantum Mechanical Model of the Atom
The Quantum Mechanical Model of the Atom Quantum Numbers In order to describe the probable location of electrons, they are assigned four numbers called quantum numbers. The quantum numbers of an electron are kind of like the electron’s “address”. No two electrons can be described by the exact same four quantum numbers. This is called The Pauli Exclusion Principle. • Principle quantum number: The principle quantum number describes which orbit the electron is in and therefore how much energy the electron has. - it is symbolized by the letter n. - positive whole numbers are assigned (not including 0): n=1, n=2, n=3 , etc - the higher the number, the further the orbit from the nucleus - the higher the number, the more energy the electron has (this is sort of like Bohr’s energy levels) - the orbits (energy levels) are also called shells • Angular momentum (azimuthal) quantum number: The azimuthal quantum number describes the sublevels (subshells) that occur in each of the levels (shells) described above. - it is symbolized by the letter l - positive whole number values including 0 are assigned: l = 0, l = 1, l = 2, etc. - each number represents the shape of a subshell: l = 0, represents an s subshell l = 1, represents a p subshell l = 2, represents a d subshell l = 3, represents an f subshell - the higher the number, the more complex the shape of the subshell. The picture below shows the shape of the s and p subshells: (notice the electron clouds) • Magnetic quantum number: All of the subshells described above (except s) have more than one orientation. -

Essential Questions and Objectives
Essential Questions and Objectives • How does the configuration of electrons affect the atom’s properties? You’ll be able to: – Draw an orbital diagram for an element – Determine the electron configuration for an element – Explain the Pauli exclusion principle, Aufbau principle and Hund’s Rule Warm up Set your goals and curiosities for the unit on your tracker. Have your comparison table out for a stamp Bohr v QMM What are some features of the quantum mechanical model of the atom that are not included in the Bohr model? Quantum Mechanical Model • Electrons do not travel around the nucleus of an atom in orbits • They are found in energy levels at different distances away from the nucleus in orbitals • Orbital – region in space where there is a high probability of finding an electron. Atomic Orbitals Electrons cannot exist between energy levels (just like the rungs of a ladder). Principal quantum number (n) indicates the relative size and energy of atomic orbitals. n specifies the atom’s major energy levels also called the principal energy levels. Energy sublevels are contained within the principal energy levels. Ground-State Electron Configuration The arrangement of electrons in the atom is called the electron configuration. Shows where the electrons are - which principal energy levels, which sublevels - and how many electrons in each available space Orbital Filling • The order that electrons fill up orbitals does not follow the order of all n=1’s, then all n=2’s, then all n=3’s, etc. Electron configuration for... Oxygen How many electrons? There are rules to follow to know where they go .. -

Chemical Bonding & Chemical Structure
Chemistry 201 – 2009 Chapter 1, Page 1 Chapter 1 – Chemical Bonding & Chemical Structure ings from inside your textbook because I normally ex- Getting Started pect you to read the entire chapter. 4. Finally, there will often be a Supplement that con- If you’ve downloaded this guide, it means you’re getting tains comments on material that I have found espe- serious about studying. So do you already have an idea cially tricky. Material that I expect you to memorize about how you’re going to study? will also be placed here. Maybe you thought you would read all of chapter 1 and then try the homework? That sounds good. Or maybe you Checklist thought you’d read a little bit, then do some problems from the book, and just keep switching back and forth? That When you have finished studying Chapter 1, you should be sounds really good. Or … maybe you thought you would able to:1 go through the chapter and make a list of all of the impor- tant technical terms in bold? That might be good too. 1. State the number of valence electrons on the following atoms: H, Li, Na, K, Mg, B, Al, C, Si, N, P, O, S, F, So what point am I trying to make here? Simply this – you Cl, Br, I should do whatever you think will work. Try something. Do something. Anything you do will help. 2. Draw and interpret Lewis structures Are some things better to do than others? Of course! But a. Use bond lengths to predict bond orders, and vice figuring out which study methods work well and which versa ones don’t will take time. -

Electron Configurations, Orbital Notation and Quantum Numbers
5 Electron Configurations, Orbital Notation and Quantum Numbers Electron Configurations, Orbital Notation and Quantum Numbers Understanding Electron Arrangement and Oxidation States Chemical properties depend on the number and arrangement of electrons in an atom. Usually, only the valence or outermost electrons are involved in chemical reactions. The electron cloud is compartmentalized. We model this compartmentalization through the use of electron configurations and orbital notations. The compartmentalization is as follows, energy levels have sublevels which have orbitals within them. We can use an apartment building as an analogy. The atom is the building, the floors of the apartment building are the energy levels, the apartments on a given floor are the orbitals and electrons reside inside the orbitals. There are two governing rules to consider when assigning electron configurations and orbital notations. Along with these rules, you must remember electrons are lazy and they hate each other, they will fill the lowest energy states first AND electrons repel each other since like charges repel. Rule 1: The Pauli Exclusion Principle In 1925, Wolfgang Pauli stated: No two electrons in an atom can have the same set of four quantum numbers. This means no atomic orbital can contain more than TWO electrons and the electrons must be of opposite spin if they are to form a pair within an orbital. Rule 2: Hunds Rule The most stable arrangement of electrons is one with the maximum number of unpaired electrons. It minimizes electron-electron repulsions and stabilizes the atom. Here is an analogy. In large families with several children, it is a luxury for each child to have their own room. -

Quantum Spin Systems and Their Local and Long-Time Properties Carolee Wheeler Faculty Advisor: Robert Sims
URA Project Proposal Quantum Spin Systems and Their Local and Long-Time Properties Carolee Wheeler Faculty Advisor: Robert Sims In quantum mechanics, spin is an important concept having to do with atomic nuclei, hadrons, and elementary particles. Spin may be thought of as a measure of a particle’s rotation about its axis. However, spin differs from orbital angular momentum in the sense that the particles may carry integer or half-integer quantum numbers, i.e. 0, 1/2, 1, 3/2, 2, etc., whereas orbital angular momentum may only take integer quantum numbers. Furthermore, the spin of a charged elementary particle is related to a magnetic dipole moment. All quantum mechanic particles have an inherent spin. This is due to the fact that elementary particles (such as photons, electrons, or quarks) cannot be divided into smaller entities. In other words, they cannot be viewed as particles that are made up of individual, smaller particles that rotate around a common center. Thus, the spin that elementary particles carry is an intrinsic property [1]. An important characteristic of spin in quantum mechanics is that it is quantized. The magnitude of spin takes values S = h s(s + )1 , with h being the reduced Planck’s constant and s being the spin quantum number (a non- negative integer or half-integer). Spin may also be viewed in composite particles, and is calculated by summing the spins of the constituent particles. In the case of atoms and molecules, spin is the sum of the spins of unpaired electrons [1]. Particles with spin can possess a magnetic moment. -

The Principal Quantum Number the Azimuthal Quantum Number The
To completely describe an electron in an atom, four quantum numbers are needed: energy (n), angular momentum (ℓ), magnetic moment (mℓ), and spin (ms). The Principal Quantum Number This quantum number describes the electron shell or energy level of an atom. The value of n ranges from 1 to the shell containing the outermost electron of that atom. For example, in caesium (Cs), the outermost valence electron is in the shell with energy level 6, so an electron incaesium can have an n value from 1 to 6. For particles in a time-independent potential, as per the Schrödinger equation, it also labels the nth eigen value of Hamiltonian (H). This number has a dependence only on the distance between the electron and the nucleus (i.e. the radial coordinate r). The average distance increases with n, thus quantum states with different principal quantum numbers are said to belong to different shells. The Azimuthal Quantum Number The angular or orbital quantum number, describes the sub-shell and gives the magnitude of the orbital angular momentum through the relation. ℓ = 0 is called an s orbital, ℓ = 1 a p orbital, ℓ = 2 a d orbital, and ℓ = 3 an f orbital. The value of ℓ ranges from 0 to n − 1 because the first p orbital (ℓ = 1) appears in the second electron shell (n = 2), the first d orbital (ℓ = 2) appears in the third shell (n = 3), and so on. This quantum number specifies the shape of an atomic orbital and strongly influences chemical bonds and bond angles. -

Lecture 3. the Origin of the Atomic Orbital: Where the Electrons Are
LECTURE 3. THE ORIGIN OF THE ATOMIC ORBITAL: WHERE THE ELECTRONS ARE In one sentence I will tell you the most important idea in this lecture: Wave equation solutions generate atomic orbitals that define the electron distribution around an atom. To start we need to simplify the math by switching to spherical polar coordinates (everything = spherical) rather than Cartesian coordinates (everything = at right angles). So the wave equations generated will now be of the form ψ(r, θ, Φ) = R(r)Y(θ, Φ) where R(r) describes how far out on a radial trajectory from the nucleus you are. r is a little way out and is small Now that we are extended radially on ψ, we need to ask where we are on the sphere carved out by R(r). Think of blowing up a balloon and asking what is going on at the surface of each new R(r). To cover the entire surface at projection R(r), we need two angles θ, Φ that get us around the sphere in a manner similar to knowing the latitude & longitude on the earth. These two angles yield Y(θ, Φ) which is the angular wave function A first solution: generating the 1s orbit So what answers did Schrodinger get for ψ(r, θ, Φ)? It depended on the four quantum numbers that bounded the system n, l, ml and ms. So when n = 1 and = the solution he calculated was: 1/2 -Zr/a0 1/2 R(r) = 2(Z/a0) *e and Y(θ, Φ) = (1/4π) . -

Chem 1A Chapter 7 Exercises
Chem 1A Chapter 7 Exercises Exercises #1 Characteristics of Waves 1. Consider a wave with a wavelength = 2.5 cm traveling at a speed of 15 m/s. (a) How long does it take for the wave to travel a distance of one wavelength? (b) How many wavelengths (or cycles) are completed in 1 second? (The number of waves or cycles completed per second is referred to as the wave frequency.) (c) If = 3.0 cm but the speed remains the same, how many wavelengths (or cycles) are completed in 1 second? (d) If = 1.5 cm and the speed remains the same, how many wavelengths (or cycles) are completed in 1 second? (e) What can you conclude regarding the relationship between wavelength and number of cycles traveled in one second, which is also known as frequency ()? Answer: (a) 1.7 x 10–3 s; (b) 600/s; (c) 500/s; (d) 1000/s; (e) ?) 2. Consider a red light with = 750 nm and a green light = 550 nm (a) Which light travels a greater distance in 1 second, red light or green light? Explain. (b) Which light (red or green) has the greater frequency (that is, contains more waves completed in 1 second)? Explain. (c) What are the frequencies of the red and green light, respectively? (d) If light energy is defined by the quantum formula, such that Ep = h, which light carries more energy per photon? Explain. 8 -34 (e) Assuming that the speed of light, c = 2.9979 x 10 m/s, Planck’s constant, h = 6.626 x 10 J.s., and 23 Avogadro’s number, NA = 6.022 x 10 /mol, calculate the energy: (i) per photon; (ii) per mole of photon for green and red light, respectively. -

Unit 3 Electrons and the Periodic Table
UNIT 3 ELECTRONS AND THE PERIODIC TABLE Chapter 5 & 6 CHAPTER 5: ELECTRONS IN ATOMS WHAT ARE THE CHEMICAL PROPERTIES OF ATOMS AND MOLECULES RELATED TO? • ELECTRONS! • Number of valence electrons • How much an atom wants to “gain” or “lose” electrons • How many electrons “gained” or “lost” ELECTRON HISTORY • Dalton’s Model • Didn’t even know of electrons or charge – just ratios of atoms. • Thomson’s Model • ELECTRONS EXIST!!!! • Where are they? No clue. • Plum Pudding? • Rutherford’s Model • Gold Foil Experiment • Electrons are found outside the nucleus. • Now knows where they are, but still doesn’t know how they are organized. Rutherford’s model BOHR’S MODEL • Electron’s are found in energy levels. Quantum: Specific energy values that have exact values in between them. • Pictured the atom like a solar system with electrons “orbiting” around the nucleus. • Each orbit path associated with a particular energy. • Now a better understanding of how electrons are organized, but it still isn’t quite correct. QUANTUM MECHANICAL MODEL ERWIN SCHRODINER (1926) • Electrons are found in regions of empty space around the nucleus. “electron clouds”. • Shape of the region corresponds to different energy levels. • Shape of the region represents the probability of the electron’s location 90% of the time. • PROBLEM? These are still called “atomic orbitals”. SCHRODINGER’S MODEL • Electrons are “wave functions”. • We can’t actually know exactly where an electron is at any moment. • We can know a probability of an electrons location based on its energy. • Ex: a windmill blade ATOMIC ORBITALS • Shape describing the probability of finding an electron at various locations around the nucleus. -
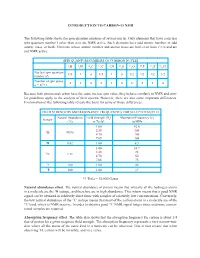
INTRODUCTION to CARBON-13 NMR the Following Table Shows The
INTRODUCTION TO CARBON-13 NMR The following table shows the spin quantum numbers of several nuclei. Only elements that have a nuclear spin quantum number I other than zero are NMR active. Such elements have odd atomic number, or odd atomic mass, or both. Elements whose atomic number and atomic mass are both even have I = 0 and are not NMR active. SPIN QUANTUM NUMBERS OF COMMON NUCLEI 1 2 12 13 14 16 17 19 31 35 1H 1H 6C 6C 7N 8O 8O 9F 15P 17Cl Nuclear spin quantum 1/2 1 0 1/2 1 0 5/2 1/2 1/2 3/2 number (I) Number of spin states 2 3 0 2 3 0 6 2 2 4 n = 2I + 1 Because both proton and carbon have the same nuclear spin value, they behave similarly in NMR and simi- lar guidelines apply to the analysis of their spectra. However, there are also some important differences. Examination of the following table reveals the basis for some of those differences. FIELD STRENGTHS AND RESONANCE FREQUENCIES FOR SELECTED NUCLEI Natural Abundance Field Strength (B ) Absorption Frequency (n) Isotope o (%) in Tesla* in MHz 1.00 42.6 2.35 100 1H 99.98 4.70 200 7.05 300 2H 0.02 1.00 6.5 1.00 10.7 2.35 25 13C 1.11 4.70 50 7.05 75 19F 100 1.00 40 31P 100 1.00 17 *1 Tesla = 10,000 Gauss Natural abundance effect. The natural abundance of proton means that virtually all the hydrogen atoms in a molecule are the 1H isotope, and therefore are in high abundance.