Experimental Alcohol-Related Peripheral Neuropathy: Role of Insulin/IGF Resistance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiamine for Prevention and Treatment of Wernicke-Korsakoff Syndrome in People Who Abuse Alcohol (Review) Copyright © 2013 the Cochrane Collaboration
Thiamine for prevention and treatment of Wernicke- Korsakoff Syndrome in people who abuse alcohol (Review) Day E, Bentham PW, Callaghan R, Kuruvilla T, George S This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2013, Issue 7 http://www.thecochranelibrary.com Thiamine for prevention and treatment of Wernicke-Korsakoff Syndrome in people who abuse alcohol (Review) Copyright © 2013 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 3 METHODS ...................................... 4 RESULTS....................................... 6 DISCUSSION ..................................... 7 AUTHORS’CONCLUSIONS . 8 ACKNOWLEDGEMENTS . 9 REFERENCES ..................................... 9 CHARACTERISTICSOFSTUDIES . 10 DATAANDANALYSES. 14 Analysis 1.1. Comparison 1 Thiamine (any dose) vs thiamine 5mg/day, Outcome 1 performance on a delayed alternation test. ..................................... 15 APPENDICES ..................................... 15 WHAT’SNEW..................................... 18 HISTORY....................................... 19 CONTRIBUTIONSOFAUTHORS . 19 DECLARATIONSOFINTEREST . 19 SOURCESOFSUPPORT . 20 INDEXTERMS .................................... 20 Thiamine for prevention and treatment of Wernicke-Korsakoff -

291.89 Other 291.9 Unspecified Alcohol-Induced Mental Disorders 303.00 Acute Alcoholic Intoxication, Unspecified 303.01 Acut
H C 116 Aortic and peripheral arterial embolism or thrombosis 291.89 Other UP ANDFACTS FIGURES: STATISTICS (arterial blood clots) 291.9 Unspecified alcohol-induced mental disorders 117 Other circulatory disease (other blood vessel disease) 303.00 Acute alcoholic intoxication, unspecified 119 Varicose veins of lower extremity (varicose veins in leg) 303.01 Acute alcoholic intoxication, continuous 120 Hemorrhoids 303.02 Acute alcoholic intoxication, episodic 121 Other diseases of veins and lymphatics (lymph system) 303.03 Acute alcoholic intoxication, in remission 303.90 Other and unspecified alcohol dependence, unspecified EXHIBIT 2.6 303.91 Other and unspecified alcohol dependence, continuous Diabetes CCS categories: 303.92 Other and unspecified alcohol dependence, episodic 49 Diabetes mellitus without complication 303.93 Other and unspecified alcohol dependence, in remission O 50 Diabetes mellitus with complications 305.00 Alcohol abuse, unspecified N ho 305.01 Alcohol abuse, continuous SPITA EXHIBIT 2.7 305.02 Alcohol abuse, episodic L - 305.03 Alcohol abuse, in remission B Pressure sore ICD-9-CM codes: ASED CARE IN T 707.0 Decubitus ulcer 357.5 Alcoholic polyneuropathy 707.00 Decubitus ulcer, unspecified site 425.5 Alcoholic cardiomyopathy 707.01 Decubitus ulcer, elbow 535.3 Alcoholic gastritis 707.02 Decubitus ulcer, upper back 535.31 Alcoholic gastritis, with hemorrhage H 707.03 Decubitus ulcer, lower back 571.0 Alcoholic fatty liver E UNITED 2005 STATES, 707.04 Decubitus ulcer, hip 571.1 Acute alcoholic hepatitis 707.05 Decubitus -

Human Alcohol-Related Neuropathology
Acta Neuropathol (2014) 127:71–90 DOI 10.1007/s00401-013-1233-3 REVIEW Human alcohol-related neuropathology Suzanne M. de la Monte · Jillian J. Kril Received: 15 October 2013 / Revised: 12 December 2013 / Accepted: 13 December 2013 / Published online: 27 December 2013 © Springer-Verlag Berlin Heidelberg 2013 Abstract Alcohol-related diseases of the nervous sys- on glia, myelin, and the microvasculature. Alcohol also tem are caused by excessive exposures to alcohol, with has devastating neurotoxic and teratogenic effects on the or without co-existing nutritional or vitamin deficiencies. developing brain in association with fetal alcohol spectrum Toxic and metabolic effects of alcohol (ethanol) vary with disorder/fetal alcohol syndrome. Alcohol impairs function brain region, age/developmental stage, dose, and duration of neurons and glia, disrupting a broad array of functions of exposures. In the mature brain, heavy chronic or binge including neuronal survival, cell migration, and glial cell alcohol exposures can cause severe debilitating diseases (astrocytes and oligodendrocytes) differentiation. Further of the central and peripheral nervous systems, and skele- progress is needed to better understand the pathophysiol- tal muscle. Most commonly, long-standing heavy alcohol ogy of this exposure-related constellation of nervous sys- abuse leads to disproportionate loss of cerebral white mat- tem diseases and better correlate the underlying pathology ter and impairments in executive function. The cerebellum with in vivo imaging and biochemical -
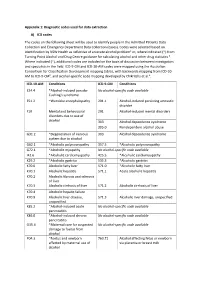
Diagnostic Codes Used for Data Extraction A) ICD Codes the Codes on the Following Sheet Will Be Used to Identify
Appendix 1: Diagnostic codes used for data extraction A) ICD codes The codes on the following sheet will be used to identify people in the Admitted Patients Data Collection and Emergency Department Data Collection (cases). Codes were selected based on identification by NSW Health as reflective of an acute alcohol problem1 or, where indicated (*) from Turning Point Alcohol and Drug Centre guidance for calculating alcohol and other drug statistics 2. Where indicated (~), additional codes are included on the basis of discussion between investigators and specialists in the field. ICD-9-CM and ICD-10-AM codes were mapped using the Australian Consortium for Classification Development mapping tables, with backwards mapping from ICD-10- AM to ICD-9-CM3, and alcohol-specific code mapping developed by Chikritzhs et al.4. ICD-10-AM Conditions ICD-9-CM Conditions E24.4 *Alcohol-induced pseudo- No alcohol-specific code available Cushing's syndrome E51.2 ~Wernicke encephalopathy 291.1 Alcohol-induced persisting amnestic disorder F10 Mental and behavioural 291 Alcohol-induced mental disorders disorders due to use of alcohol 303 Alcohol dependence syndrome 305.0 Nondependent alcohol abuse G31.2 *Degeneration of nervous 303 Alcohol dependence syndrome system due to alcohol G62.1 *Alcoholic polyneuropathy 357.5 *Alcoholic polyneuropathy G72.1 *Alcoholic myopathy No alcohol-specific code available I42.6 *Alcoholic cardiomyopathy 425.5 *Alcoholic cardiomyopathy K29.2 *Alcoholic gastritis 535.3 *Alcoholic gastritis K70.0 Alcoholic fatty liver 571.0 *Alcoholic -

The Consumption and Consequences of Alcohol and Drugs in Allen County a County Epidemiological Profile 2009
The Consumption and Consequences of Alcohol and Drugs in Allen County A County Epidemiological Profile 2009 Drug & Alcohol Consortium of Allen County 532 West Jefferson Blvd Fort Wayne, IN 46802 (260) 422-8412 Fax: (260) 423-1733 [email protected] www.dacac.org The Consumption and Consequences of Alcohol and Drugs in Allen County: A County Epidemiological Profile 2009 Developed by the Research/Local Epidemiology and Outcomes Workgroup, 2009 Our Vision ∗ A community culture of invested organizations replicating their own SPF activities at the front lines ∗ All primary, secondary, and higher education to install evidence- based prevention into their curricula ∗ Serving, educating, enjoining, and facilitating targeted corporations to install evidence-based prevention and intervention programs ∗ A community intolerance of under-aged and binge drinking by youth ∗ A 50% reduction in under-aged and binge drinking by 2020 Our Mission To reduce substance use and abuse among youth and young adults in Allen County. Published by the Drug & Alcohol Consortium of Allen County 2 Allen County Strategic Prevention Framework Advisory Council Paula Hughes, Chair Deborah McMahan, M.D. Council Member, District 2 Health Commissioner Allen County Council Allen County – Fort Wayne Department of Health Dick Conklin Sharon Mejeur Executive Director Vice-President of Student Life Tobacco Free Allen County University of St. Francis Andrew Downs, PhD Wyatt Mullinax Director Commissioner, Drug-Free Indiana Mike Downs Center for Indiana Politics Indiana Criminal Justice Institute Indiana-Purdue University at Fort Wayne Honorable Thomas Felts Marty Pastura Judge President/CEO Allen County Circuit Court YMCA of Greater Fort Wayne Loren Fifer Jerry Peterson District 4 Representative, Vice-President President/CEO Indiana Beverage Commission United Way of Allen County Kenneth Fries Denise Porter-Ross Sheriff Mayor’s Northeast Area Advocate Allen County Sheriff’s Department City of Fort Wayne Honorable Frances Gull Kirk Ray Judge Chief Executive Officer Allen Superior Court St. -

Jemds.Com Original Research Article
Jemds.com Original Research Article A CROSS- SECTIONAL STUDY ON CLINICAL PROFILE OF ALCOHOL INDUCED NEUROLOGICAL MANIFESTATIONS Indira Priyadarsini Chandra1, Sreedevi Boppudi2, S. Sai Charitha3, B. Vyshnavi4, N. Keerthi5 1Associate Professor, Department of General Medicine, ACSR Government Medical College, Nellore, Andhra Pradesh, India. 2Associate Professor, Department of Community Medicine, ACSR Government Medical College, Nellore, Andhra Pradesh, India. 3Pharmacy Student, Sri Padmavathi School of Pharmacy, Tirupati, Andhra Pradesh, India. 4Pharmacy Student, Sri Padmavathi School of Pharmacy, Tirupati, Andhra Pradesh, India. 5Pharmacy Student, Sri Padmavathi School of Pharmacy, Tirupati, Andhra Pradesh, India. ABSTRACT BACKGROUND Alcohol is the most frequently consumed toxic substance in the world. Alcohol contributes to over 200 diseases and related health conditions, most notably alcohol dependence. Globally, alcohol misuse is the fifth leading risk factor for premature death and disability among people between the ages of 15 and 49. WHO stated that in the age group 20–39 years, approximately 25 percent of the total deaths were attributable to alcoholism Acute. alcohol intoxication is associated with number of complications including accidents and domestic violence. During drinking periods and withdrawal, alcoholics commonly experience sleep disturbances like falling asleep and staying asleep (decreased total sleep time). Some neurological disorders related to long term alcoholism are predominantly due to inadequate nutrition (thiamine deficiency that causes Wernicke's encephalopathy), but others appear to involve the neurotoxicity of ethanol on brain (alcohol withdrawal syndrome and dementia) and peripheral nerves (alcoholic neuropathy and myopathy).1 We wanted to study the effect of alcohol on central and peripheral nervous system in alcohol abuse patients admitted to Sri Venkateswara Ramnarayan Ruia, Government General Hospital, Tirupati. -

Alcohol and Health: TABLECONTENTS of CONTENTS
TABLE OF Alcohol and Health: TABLECONTENTS OF CONTENTS Current Evidence NOV-DEC 2006 ALCOHOL AND HEALTH OUTCOMES Alcohol and Health Outcomes Does Inflammation Influence Alcohol’s Cardiovascular Does Inflammation Influence Alcohol’s Cardiovascular Effects? Effects?, 1 Light-to-moderate alcohol use can reduce women had an outcome event. cardiovascular mortality in some popula- Long-Term Mortality in People tions. To investigate whether this protective Comments: This interesting research is con- Treated for Alcoholism, 1 effect is influenced by inflammation, re- sistent with prior studies that show re- searchers assessed alcohol use and inflamma- duced all-cause mortality and cardiac events tory markers (C-reactive protein and inter- in adults who drink light-to-moderate Moderate Drinking Impairs the Ability to See, 2 leukin-6) in 2487 adults, aged 70–79 years, amounts. Although the study found no rela- without heart disease at study entry. Over a tionship between C-reactive protein levels, mean 5.6 years of follow-up, 397 deaths and alcohol use, and outcomes, it did find a Alcohol and Cancer Worldwide, 3 383 cardiac events (myocardial infarction, lower risk in light-to-moderate drinking angina, or heart failure) occurred. men with high (but not low) interleukin-6 levels. To better understand the interaction Alcohol-Attributable Mortality and Morbidity in Canada, 3 • In adjusted analyses, the risks of all- of inflammation, alcohol, and cardiovascular cause mortality and incident cardiac health, further research on this topic events were lower in light-to-moderate should include different populations, such Prescription Drug Misuse Is drinkers* than in never or occasional as people with chronic inflammatory condi- More Common in Drinkers, 3 drinkers** (hazard ratios [HRs] 0.7 for tions, women, and racial minorities. -

Supplementary File ICD Codes
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Br J Sports Med ICD-8 ICD-9 ICD-10 Code Description Code Description Code Description 291 Alcoholic psychosis 291 Alcoholic psychosis F10 Use of alcohol Psychosis associated with drug or 294.3 292 Drug psychosis F11 Use of opioids poison 303 Alcoholism 303 Alcohol dependence syndrome F12 Use of cannabinoids 393 Other/unspecified alcoholism 304 Drug dependence F13 Use of sedatives or hypnotics 304 Drug dependence 305 Drug abuse F14 Use of cocaine Use of other stimulants including 571 Alcoholic cirrhosis 357.5 Alcoholic polyneuropathy F15 caffeine 357.6 Drugs polyneuropathy F16 Use of hallucinogens Substance 425.5 Alcoholic cardiomyopathy F17 Use of tobacco Abuse 535.3 Alcoholic gastritis F18 Use of volatile solvents Multiple drug use or use of other 571.0 Alcoholic fatty liver F19 psychoactive substances 571.1 Acute alcoholic hepatitis G62.0 Alcoholic polyneuropathy 571.2 Alcohol cirrhosis of liver G62.1 Drug-induced polyneuropathy 571.3 Alcohol liver damage unspecified I42.6 Alcoholic cardiomyopathy Alcoholic gastritis without K29.20 bleeding K70 Alcoholic liver disease 306.5 Feeding disturbance 307.1 Anorexia nervosa F50 Eating disorders Eating 307.5 Other eating disorders R63.0 Anorexia Disorders 783 Anorexia 296 Affective psychoses 296 Affective psychoses F30 Manic episodes 298 Depressive psychoses 298 Depressive psychoses F31 Bipolar -

Alcohol (Ethanol) Related Neuropathy : Article by Tarakad S Ramachandran 9/9/08 10:54 PM
eMedicine - Alcohol (Ethanol) Related Neuropathy : Article by Tarakad S Ramachandran 9/9/08 10:54 PM J Brock Account Settings Log Out HOME SPECIALTIES REFERENCE CENTERS Search: eMedicine Clinical Reference, Drug Reference, MEDLINE, and more Search You are in: eMedicine Specialties > Neurology > Neurotoxicology Quick Find Alcohol (Ethanol) Related Neuropathy Email to a colleague Authors & Editors Introduction Clinical Article Last Updated: Jul 10, 2006 Differentials Section 1 of 8 Workup AUTHOR AND EDITOR INFORMATION Treatment Follow-up Authors and Editors Introduction Clinical Differentials Workup Treatment Follow-up References References Related Articles Author: Tarakad S Ramachandran, MBBS, FRCP(C), FACP, Chief, Department of Neurology, Amyotrophic Lateral Crouse Irving Memorial Hospital; Professor, Department of Neurology, State University of New York Sclerosis Upstate Medical University Chronic Inflammatory Demyelinating Tarakad S Ramachandran is a member of the following medical societies: American Academy of Polyradiculoneuropathy Clinical Electroencephalographers, American Academy of Neurology, American Academy of Pain Medicine, American College of Forensic Examiners, American College of Managed Care Medicine, Diabetic Neuropathy American College of Physicians, Royal College of Physicians, Royal College of Physicians and Femoral Mononeuropathy Surgeons of Canada, Royal College of Surgeons of England, and Royal Society of Medicine HIV-1 Associated Acute/Chronic Coauthor(s): Charles Gellido, MD, Laboratory Director, Assistant Professor, -

Prognosis of Alcoholic Peripheral Neuropathy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.47.7.699 on 1 July 1984. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1984;47:699-703 Prognosis of alcoholic peripheral neuropathy MATTI HILLBOM,* ARNE WENNBERGt From the Department of Clinical Alcohol and Drug Research, * Karolinska Hospital, Stockholm, National Board ofOccupational Safety and Health, Research Department, Solna, t and Department of Clinical Neurophysiology, t Karolinska Hospital, Stockholm, Sweden SUMMARY Ten male alcoholics aged 38-72 years with clear clinical and electroneurographical signs of peripheral neuropathy were re-examined three to five years later. Conduction velocities, latencies and nerve action potential amplitudes were measured from median, peroneal and sural nerves on both occasions and the results were compared with age-matched reference values from 80 healthy men. Seven of the alcoholics showed normal or nearly normal scores in electroneurog- raphical and clinical examination and they had all managed to stop drinking alcohol. The results suggest that the prognosis of alcoholic peripheral neuropathy is good and independent of age provided that intake of alcohol is discontinued and other causes of neuropathy (malignancy, diabetes, nerve trauma) are carefully excluded. The data collected so far have failed to establish the by direct questioning, measurement of breath alcohol and Protected by copyright. prognosis of alcoholic polyneuropathy.'-4 This may assay of various enzymes (GGT, ASAT, ALAT) and mean be due to frequent dropout of alcoholics from their corpuscular volume. rehabilitation programmes and poor diagnostic Electroneurography was performed on three nerves at accuracy. We were able to follow up a small group of both occasions: median, peroneal and sural nerves, and the and electroneurog- following 10 electroneurographical parameters were alcoholics with clear clinical measured: Median nerve: (1) Motor nerve conduction vel- raphical findings of peripheral neuropathy. -
Review Article Alcoholic Neuropathy and Remedies
Journal of Innovations in Pharmaceuticals and Biological Sciences JIPBS www.jipbs.com ISSN: 2349-2759 Review article Alcoholic neuropathy and remedies Shashidhar Mehta*1, Puneet Sudan2, Shaveta Bhardwaj3, Sonika Redhu Sihag4, Rajesh Kumar Pandey5 1Department of Biochemistry, Mewar University, Rajasthan. 2Chandigarh College of Pharmacy, Landran, Punjab. 3Department of Pharmacy, LLR College of Pharmacy, Moga, Punjab. 4Shekhwati College of Pharmacy, Dunload, Jhunjhunu, Rajasthan. 5All Excel Inc., 135 Wood Street Suite 201 West Haven, CT 06516. Abstract Neuropathy is caused by a wide variety of factors, however majority of them are under reported till date. Medicinal plants have been used in research long before the centuries to extract out the therapeutic effect in order to relieve the morbidities. The administration of alcohol for long time causes the advent of alcoholic neuropathic like state. Herbal drugs offer a novel approach in the treatment of the disorder. Thus, further exploration is needed in order to explore the further mechanism that will provide substantial support for claiming the therapeutic effect. Key words: neuropathy; chronic; alcoholism; remedies; immunity *Corresponding Author: Shashidhar Mehta, Department of Biochemistry, Mewar University, Rajasthan. 1. Introduction Neuropathy is caused by a number of the condition of the neuropathy worsens to factors including heredity causes, induction the utmost level when a person is suffering of diabetes like morbidity, advent of from fever or any other disorder that leads arthritis problem, HIV and cancer disorders to loss of immunity occurs because that and induction of heavy metals like arsenic, condition destroys the myelin sheath that lead and mercury. The ingestion of alcohol provides protective covering of the nerves causes the problem of neuropathy to a and neurons. -
Alcohol-Related Neurological Disease
Alcohol-related neurological disease Definition Alcohol-related neurological disease represents a broad spectrum of conditions caused by acute or chronic alcohol intake. Description Alcohol, or ethanol, is a poisonous chemical that has direct and toxic effects on nerve and muscle cells. The effects can be profound, and symptoms can include incoordination, weakness, seizures , memory loss, and sensory deficits. Alcohol has a profoundly negative effect on both the central nervous system (i.e., the brain and spinal cord) and the peripheral nervous system (i.e., nerves that send impulses to peripheral structures such as muscles and organs). Alcohol can have negative effects on neurological centers that regulate body temperature, sleep, and coordination. Alcohol can significantly lower body temperature. It disrupts normal sleep patterns because it decreases rapid eye movement (REM) during the dreaming stage of sleep. It also adversely affects muscle coordination, causing imbalance and staggering—alcohol is a toxic insult to the cerebellum , which is responsible for balance. Additionally, the chronic use of alcohol can cause a broad spectrum of abnormalities in mental functioning. Generally, persons exhibit poor attention, difficulty with abstraction and problem solving, difficulty learning new materials, reduced visuospatial abilities (capacity to discriminate between two- dimensional or three-dimensional space), and often require extra time to integrate visual information. Other related problems include thiamine deficiency (vitamin B-1) and liver disease (liver cirrhosis and possibly liver cancer). Acute effects of alcohol When alcohol is ingested, it moves from the bloodstream into every part of the body that contains water, including the brain, lungs, kidneys, and heart. Alcohol distributes itself equally both inside and outside cells.