Susceptibility of Larvae of Nun Moth, Lymantria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Main Characteristic Features of the Bulgarian Orlitza Dialect (From the East Rhodopes)
US-China Foreign Language, June 2015, Vol. 13, No. 6, 417-422 doi:10.17265/1539-8080/2015.06.003 D DAVID PUBLISHING The Main Characteristic Features of the Bulgarian Orlitza Dialect (From the East Rhodopes) Ivan Georgiev Iliev Plovdiv University, Plovdiv, Bulgaria This article describes the main characteristic features of the dialect spoken by Muslim Bulgarians inhabiting the East Rhodopian Bulgarian village of Orlitza. The dialect features phonetic changes © Æ a, ú Æ a, ќ Æ ’a, ü Æ ’a; the traces from the Old Bulgarian nasal vowels; the use of a definite article from the Old Bulgarian demonstrative pronoun òú “this” unlike most of the other Rhodopian dialects where a triple article derived from the demonstrative pronouns òú, ñü, îíú is in use; as well as interesting lexemes, often from Turkish origin. Кeywords: Bulgarian language, Bulgarian dialectology, East Rhodopes Introduction The village of Orlitza, within the municipality of Kirkovo, is situated near the Greek-Bulgarian border, not far from the Makaza pass (and the border check point with the same name). The inhabitants of the village are Muslim Bulgarians (Pomaks).1 Their dialect is almost unknown. In his book on the Tihomir dialect, Kabasanov (1963) mentions in passing several basic characteristic features of the dialectal varieties spoken in the villages of Lozengradtzi, Strizhba, Tzarinovo, and Orlitza (called Protogerovo at that time): the transition © Æ а, the traces of nasal vowels, the rare change o Æ a, the prefixal change пир- Æ при-. So far, only short articles concerning the examined here dialect of Orlitza have been published in local newspapers (Kostovska, 1971; Avdzhiev, 1988). -

Report by Institute of Viticulture and Enology, Pleven
REPORT BY INSTITUTE OF VITICULTURE AND ENOLOGY, PLEVEN BY ACTIVITY 3.2.1 .: DESCRIPTION OF WINE GRAPE VARIETIES AND MICRO AREAS OF PRODUCTION IN THE HASKOVO AND KARDZHALI DISTRICTS OCTOBER, 2018 This report was prepared by a team of scientists from the Institute of Viticulture and Enology, Pleven, Bulgaria for the purpose of the project DIONYSOS. The analysis of the report uses own research; references to scientific literature in the field of viticulture, wine, history, geography, soil science, climate and tourism of bulgarian and world scientists; official statistics of NSI, MAFF, NIMH; officially published documents such as districts and municipalies development strategies in the districts of Haskovo and Kardzhali; the Law on Wine and Spirits of the Republic of Bulgaria; the Low of Tourism of the Republic of Bulgaria; official wine cellar websites, tourist information centers, travel agencies; and other sources. This document is created under the project “Developing identity on yield, soil and site”/DIONYSOS, Subsidy contract B2.6c.04/01.11.2017 with the financial support of Cooperation Programme “Interreg V-A Greece-Bulgaria” 2014-2020, Co- funded by the European Regional Development Fund and National funds of Greece and Bulgaria. The entire responsibility for the contents of the document rests with Institute of Viticulture and Enology-Pleven and under no circumstances it can be assumed that the materials and information on the document reflects the official view European Union and the Managing Authority Този документ е създаден в рамките на проект „Разработване на идентичност на добива, почвите и местностите“/ДИОНИСОС, Договор за субсидиране B2.6c.04/01.11.2017 който се осъществява с финансовата подкрепа на подкрепа на Програма за трансгранично сътрудничество ИНТЕРРЕГ V-A Гърция-България 2014-2020, съфинансирана от Европейския фонд за регионално развитие и от националните фондове на страните Гърция и България. -

Annexes to Rural Development Programme
ANNEXES TO RURAL DEVELOPMENT PROGRAMME (2007-2013) TABLE OF CONTENTS Annex 1 ...........................................................................................................................................4 Information on the Consultation Process ........................................................................................4 Annex 2 .........................................................................................................................................13 Organisations and Institutions Invited to the Monitoring Committee of the Implementation of the Rural Development Programme 2007-2013 .................................................................................13 Annex 3 .........................................................................................................................................16 Baseline, Output, Result and Impact Indicators............................................................................16 Annex 4 .........................................................................................................................................29 Annexes to the Axis 1 Measures...................................................................................................29 Attachment 1 (Measure 121 Modernisation of Agricultural Holding) .........................................30 List of Newly Introduced Community Standards .........................................................................30 Attachment 1.A. (Measure 121 Modernisation of Agricultural Holding -

REGISTER for Transport of Animals During Short Journeys Under Article 165 of LVA
REGISTER for transport of animals during short journeys under article 165 of LVA № and date of certificate of № and date of competence for Number the transporter address of name/identificat identification of Types of date of changes of № drivers and of transporter identification transporter ion of company ransport vehicle animals expiry data attendants animals authorisation under Art. 164 of LVA 172 0172/15.10.09 Blagoy Borisov Razlog town, 77 Blagoy Borisov Stayer 11 C 18 with 19/ Lardge 13 This Velev Ekzarh Yosiv str. Velev reg. № E9413BP, 09.03.2009 ruminants vichicle is total area 24 m² Small 100 didn’t use ruminants any more Pigs 50 because is Horses 8 disposed. Volvo with 19/ Large 10 reg.№E2904KK with 09.03.2009 ruminants total area 14 m² Small 84 ruminants Horses 8 pigs 32 Yordan Ivanov Volvo with 38/25.04.2013 Large 10 Saev reg.№6847BX with ruminants total area 15 m² Small 90 ruminants Horses 9 pigs 35 174 0174/15.10.09 Stoyan Lybenov Region of Stoyan Lybenov Mercedes 410 D with 20/ Lardge 9 Savov Blagoevgrad, Savov reg. № E9025BC, 28.04.2009 ruminants Musomishta vilage total area 12 m² Small 45 ruminants 175 0175/15.10.09 Vasil Petrov Region of Vidin, Radoslav Velkov Nissan vannet with 5 Day old birds 2250 Kamen village Bela Rada Petrov – rent reg. № CA5151HT, 12.08.2009 <1,6 kg 180 total area 93,08 m² 1,6 – 3 kg 90 176 0176/22.10.09 SVINEKOMLE Region of Ruse, SVINEKOMLE Ford Transit with reg. 11/ Pigs X – YUDELNIK Yudelnik vilage X – YUDELNIK № P8724PC, total 20.10.2009 <25 kg 40 Ltd. -

REGISTER for Transport of Animals During Short Journeys Under Article 165 of LVA Update: 06.01.2017
REGISTER for transport of animals during short journeys under article 165 of LVA Update: 06.01.2017 № and date of certificate of № and date of competence for Number the transporter address of name/identificat identification of Types of date of changes of № drivers and of transporter identification transporter ion of company ransport vehicle animals expiry data attendants animals authorisation under Art. 164 of LVA 402. 0402/16.01.2012 “Dage-Dako Montana region, ”AFIN- Iveko 65C15 with XO Live fish 1 200kg. Dachev” St. Lom city, №53 Bulgaria” Ltd, reg. №CA8056MT 0031/01.12.20 ”Slavyanska ” str. Sofia, №26A, with total area 8,40 10 Bl. Almus , entr. B, ”Raiko м² app. 3 Aleksiev” str. With contract 403. 0403/21.01.2012 Petya Dinkova Sofia city, kv. Petya Dinkova MAN 8.100 with reg. 12/06.01.2012 Large 10 Krasteva Lozenetc, №14 Krasteva №EH6495BM with ruminants ”Buntovnik” str. total area 13 м² Small 32 ruminants pigs 26 404. 0404/21.01.2012 “LIN- Iliyana Montana city, kv. “LIN- Iliyana Mitsubishi L 200 0188/19.10.20 Live fish 200 kg. Nikolova” Ltd Pliska №18, entr. Nikolova” Ltd with reg. 11 A, app. 1 №M8944AP with total area 3.04 м² 405. 0405/21.01.2012 Ahmed Piperkovo village, Ahmed Ford Tranzit with 23/14.12.2011 Large 5 Yasharov Tsenovo Yasharov reg. №P3719AX ruminants Ashimov municipality, Ruse Ashimov with total area 7 м² Small 17 region ruminants pigs 16 406. 0406/21.01.2012 “Almerta” Ltd Kardzhali city, kv. “Almerta” Ltd Fiat Ducato with reg. 44/18.01.2012 Large 6 Vazrozhdentsi, bl. -
List of Appendices
Preparation of Regional Water and Wastewater Master Plans for Central Region Regional Final Master Plan for ViK OOD - Kardzhali LIST OF APPENDICES APPENDIX 1-1 PROJECTS UNDER IMPLEMENTATION ............................................................................................. 1 APPENDIX 1-2 REGULATORY FRAMEWORK IN BULGARIA...................................................................................... 3 APPENDIX 1-3 REGULATORY FRAMEWORK IN THE EUROPEAN COMMUNITY ....................................................... 9 APPENDIX 2-1 PROJECT SCOPE ............................................................................................................................. 12 APPENDIX 2-2 ADMINISTRATIVE DATA ................................................................................................................ 13 APPENDIX 2-3 MAPS OF GROUNDWATER BODIES ................................................................................................ 22 APPENDIX 2-4 DRINKING WATER MONITORING FOR THE YEAR 2012 ................................................................... 26 APPENDIX 3-1 GENERAL FEATURES OF SURFACE WATER...................................................................................... 28 APPENDIX 3-2 GROUNDWATER QUANTITY .......................................................................................................... 32 APPENDIX 3-3 WATER SUPPLY ZONES ................................................................................................................. 39 APPENDIX 3-4 GROUNDWATER -

Annexes to Rural Development Programme
ANNEXES TO RURAL DEVELOPMENT PROGRAMME (2007-2013) TABLE OF CONTENTS Annex 1 ...........................................................................................................................................4 Information on the Consultation Process ........................................................................................4 Annex 2 .........................................................................................................................................13 Organisations and Institutions Invited to the Monitoring Committee of the Implementation of the Rural Development Programme 2007-2013 .................................................................................13 Annex 3 .........................................................................................................................................16 Baseline, Output, Result and Impact Indicators............................................................................16 Annex 4 .........................................................................................................................................29 Annexes to the Axis 1 Measures...................................................................................................29 Attachment 1 (Measure 121 Modernisation of Agricultural Holding) .........................................30 List of Newly Introduced Community Standards .........................................................................30 Attachment 2 (Measure 123 Adding value of agricultural and forestry -
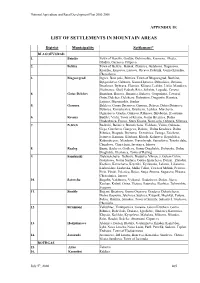
List of Settlements in Mountain Areas
National Agriculture and Rural Development Plan 2000-2006 APPENDIX 1E LIST OF SETTLEMENTS IN MOUNTAIN AREAS District Municipality Settlement* BLAGOEVGRAD 1. Bansko Town of Bansko, Gostun, Dobrinishte, Kremena, Mesta, Obidim, Osenovo, Filipovo 2. Belitza Town of Belitza , Babiak, Zlatarica, Gylybovo, Dagonovo, Kraishte, Kuzaovo, Liutovo, Orcevo, Palatnik, Gorno Kraishte, Chereshovo 3. Blagoevgrad Izgrev, Belo pole, Bistrica, Town of Blagoevgrad, Buchino, Bylgarchevo, Gabrovo, Gorno Hyrsovo, Debochica, Delvino, Drenkovo, Dybrava, Elenovo, Klisura, Leshko, Lisiia, Marulevo, Moshtanec, Obel, Padesh, Rilci, Selishte, Logodaj, Cerovo 4. Gotze Delchev Banichan, Borovo, Breznica, Bukovo, Gospodinci, Town of Gotze Delchev, Delchevo, Dobrotino, Dragostin, Kornica, Lyjnica, Musomishta, Sredna 5. Garmen Baldevo, Gorno Drianovo, Garmen, Debren, Dolno Drianovo, Dybnica, Kovachevica, Krushevo, Leshten, Marchevo, Ognianovo, Oreshe, Osikovo, Ribnovo, Skrebatno, Hvostiane 6. Kresna Budilci, Vlahi, Town of Kresna, Gorna Breznica, Dolna Gradeshnica, Ezerec, Stara Kresna, Novo selo, Oshtava, Slivnica 7. Petrich Baskalci, Belasica, Borovichene, Vishlene, Volno, Gabrene, Gega, Gorchevo, Giurgevo, Dolene, Dolna Krushica, Dolna Ribnica, Dragush, Drenovo, Drenovica, Zanoga, Zoichene, Ivanovo, Kamena, Kladenci, Kliuch, Kolarovo, Kryndjilica, Kukurahcevo, Mendovo, Pravo byrdo, Samuilovo, Tonsko dabe, Churilovo, Churicheni, Iavornica, Iakovo 8. Razlog Bania, Bachevo, Godlevo, Gorno Draglishte, Dobyrsko, Dolno Draglishte, Eleshnica, Town of Razlog 9. Sandanski Belevehchevo,