Algorithms and Models for Protein Structure Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the Number of Unknot Diagrams Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar
On the number of unknot diagrams Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar To cite this version: Carolina Medina, Jorge Luis Ramírez Alfonsín, Gelasio Salazar. On the number of unknot diagrams. SIAM Journal on Discrete Mathematics, Society for Industrial and Applied Mathematics, 2019, 33 (1), pp.306-326. 10.1137/17M115462X. hal-02049077 HAL Id: hal-02049077 https://hal.archives-ouvertes.fr/hal-02049077 Submitted on 26 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. On the number of unknot diagrams Carolina Medina1, Jorge L. Ramírez-Alfonsín2,3, and Gelasio Salazar1,3 1Instituto de Física, UASLP. San Luis Potosí, Mexico, 78000. 2Institut Montpelliérain Alexander Grothendieck, Université de Montpellier. Place Eugèene Bataillon, 34095 Montpellier, France. 3Unité Mixte Internationale CNRS-CONACYT-UNAM “Laboratoire Solomon Lefschetz”. Cuernavaca, Mexico. October 17, 2017 Abstract Let D be a knot diagram, and let D denote the set of diagrams that can be obtained from D by crossing exchanges. If D has n crossings, then D consists of 2n diagrams. A folklore argument shows that at least one of these 2n diagrams is unknot, from which it follows that every diagram has finite unknotting number. -

On Computation of HOMFLY-PT Polynomials of 2–Bridge Diagrams
. On computation of HOMFLY-PT polynomials of 2{bridge diagrams . .. Masahiko Murakami . Joint work with Fumio Takeshita and Seiichi Tani Nihon University . December 20th, 2010 1 Masahiko Murakami (Nihon University) On computation of HOMFLY-PT polynomials December 20th, 2010 1 / 28 Contents Motivation and Results Preliminaries Computation Conclusion 1 Masahiko Murakami (Nihon University) On computation of HOMFLY-PT polynomials December 20th, 2010 2 / 28 Contents Motivation and Results Preliminaries Computation Conclusion 1 Masahiko Murakami (Nihon University) On computation of HOMFLY-PT polynomials December 20th, 2010 3 / 28 There exist polynomial time algorithms for computing Jones polynomials and HOMFLY-PT polynomials under reasonable restrictions. Computational Complexities of Knot Polynomials Alexander polynomial [Alexander](1928) Generally, polynomial time Jones polynomial [Jones](1985) Generally, #P{hard [Jaeger, Vertigan and Welsh](1993) HOMFLY-PT polynomial [Freyd, Yetter, Hoste, Lickorish, Millett, Ocneanu](1985) [Przytycki, Traczyk](1987) Generally, #P{hard [Jaeger, Vertigan and Welsh](1993) 1 Masahiko Murakami (Nihon University) On computation of HOMFLY-PT polynomials December 20th, 2010 4 / 28 Computational Complexities of Knot Polynomials Alexander polynomial [Alexander](1928) Generally, polynomial time Jones polynomial [Jones](1985) Generally, #P{hard [Jaeger, Vertigan and Welsh](1993) HOMFLY-PT polynomial [Freyd, Yetter, Hoste, Lickorish, Millett, Ocneanu](1985) [Przytycki, Traczyk](1987) Generally, #P{hard [Jaeger, Vertigan -

Mathematics and Computation
Mathematics and Computation Mathematics and Computation Ideas Revolutionizing Technology and Science Avi Wigderson Princeton University Press Princeton and Oxford Copyright c 2019 by Avi Wigderson Requests for permission to reproduce material from this work should be sent to [email protected] Published by Princeton University Press, 41 William Street, Princeton, New Jersey 08540 In the United Kingdom: Princeton University Press, 6 Oxford Street, Woodstock, Oxfordshire OX20 1TR press.princeton.edu All Rights Reserved Library of Congress Control Number: 2018965993 ISBN: 978-0-691-18913-0 British Library Cataloging-in-Publication Data is available Editorial: Vickie Kearn, Lauren Bucca, and Susannah Shoemaker Production Editorial: Nathan Carr Jacket/Cover Credit: THIS INFORMATION NEEDS TO BE ADDED WHEN IT IS AVAILABLE. WE DO NOT HAVE THIS INFORMATION NOW. Production: Jacquie Poirier Publicity: Alyssa Sanford and Kathryn Stevens Copyeditor: Cyd Westmoreland This book has been composed in LATEX The publisher would like to acknowledge the author of this volume for providing the camera-ready copy from which this book was printed. Printed on acid-free paper 1 Printed in the United States of America 10 9 8 7 6 5 4 3 2 1 Dedicated to the memory of my father, Pinchas Wigderson (1921{1988), who loved people, loved puzzles, and inspired me. Ashgabat, Turkmenistan, 1943 Contents Acknowledgments 1 1 Introduction 3 1.1 On the interactions of math and computation..........................3 1.2 Computational complexity theory.................................6 1.3 The nature, purpose, and style of this book............................7 1.4 Who is this book for?........................................7 1.5 Organization of the book......................................8 1.6 Notation and conventions..................................... -

Combinatorics for Knots
Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness Combinatorics for Knots J. Ram´ırezAlfons´ın Universit´eMontpellier 2 J. Ram´ırezAlfons´ın Combinatorics for Knots Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness 1 Basic notions 2 Matroid 3 Knot coloring and the unknotting problem 4 Oriented matroids 5 Spatial graphs 6 Ropes and thickness J. Ram´ırezAlfons´ın Combinatorics for Knots Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness J. Ram´ırez Alfons´ın Combinatorics for Knots Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness Reidemeister moves I !1 I II !1 II III !1 III J. Ram´ırez Alfons´ın Combinatorics for Knots Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness III II II I II II J. Ram´ırezAlfons´ın Combinatorics for Knots Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness III I II I J. Ram´ırez Alfons´ın Combinatorics for Knots Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness III I I II II I I J. Ram´ırez Alfons´ın Combinatorics for Knots Basic notions Matroid Knot coloring and the unknotting problem Oriented matroids Spatial graphs Ropes and thickness Bracket polynomial For any link diagram D define a Laurent polynomial < D > in one variable A which obeys the following three rules where U denotes the unknot : J. -

Using Reidemeister Moves for UNKNOTTING
Algorithms in Topology Today’s Plan: Review the history and approaches to two fundamental problems: Unknotting Manifold Recognition and Classification What are knots? Knots are closed loops in R3, up to isotopy Embedding Projection What are knots? We can study smooth or polygonal knots. These give equivalent theories, but polygonal knots are more natural for computation. What are knots? Knots are closed loops in space, up to isotopy We can study smooth or polygonal knots. These give equivalent theories, but polygonal knots are more natural for computation. For algorithmic purposes, we can explicitly describe a knot as a polygon in Z3. K = {(0,0,0), (1,2,0), (2,3,8), ... , (0,0,0)} We can also use several equivalent descriptions. Some Basic Questions • Can we classify knots? • Can we recognize a particular knot, such as the unknot? • How hard is it to recognize a knot? • Does topology say something new about complexity classes? • Do undecidable problems arise in the study of knots and 3- manifolds. • Does the study of topological and geometric algorithms lead to new insight into classical problems? (Isoperimetric inequalities, P=NP? NP=coNP?) Basic Questions about Manifolds • Can we classify manifolds? • Can we recognize a particular manifold, such as the sphere? • How hard is it to recognize a manifold? (What is the complexity of an algorithm) • What undecidable problems arise in the study of knots and 3- manifolds? • Does the study of topological and geometric algorithms lead to new insight into classical problems? (Isoperimetric inequalities, P=NP? NP=coNP?) Describing Surfaces and 3-Manifolds What type of surfaces and manifolds do we consider? There are three main categories to choose from: Smooth Piecewise Linear Continuous Describing Surfaces and 3-Manifolds The continuous theory allows for more pathological examples. -

Topics in Low Dimensional Computational Topology
THÈSE DE DOCTORAT présentée et soutenue publiquement le 7 juillet 2014 en vue de l’obtention du grade de Docteur de l’École normale supérieure Spécialité : Informatique par ARNAUD DE MESMAY Topics in Low-Dimensional Computational Topology Membres du jury : M. Frédéric CHAZAL (INRIA Saclay – Île de France ) rapporteur M. Éric COLIN DE VERDIÈRE (ENS Paris et CNRS) directeur de thèse M. Jeff ERICKSON (University of Illinois at Urbana-Champaign) rapporteur M. Cyril GAVOILLE (Université de Bordeaux) examinateur M. Pierre PANSU (Université Paris-Sud) examinateur M. Jorge RAMÍREZ-ALFONSÍN (Université Montpellier 2) examinateur Mme Monique TEILLAUD (INRIA Sophia-Antipolis – Méditerranée) examinatrice Autre rapporteur : M. Eric SEDGWICK (DePaul University) Unité mixte de recherche 8548 : Département d’Informatique de l’École normale supérieure École doctorale 386 : Sciences mathématiques de Paris Centre Numéro identifiant de la thèse : 70791 À Monsieur Lagarde, qui m’a donné l’envie d’apprendre. Résumé La topologie, c’est-à-dire l’étude qualitative des formes et des espaces, constitue un domaine classique des mathématiques depuis plus d’un siècle, mais il n’est apparu que récemment que pour de nombreuses applications, il est important de pouvoir calculer in- formatiquement les propriétés topologiques d’un objet. Ce point de vue est la base de la topologie algorithmique, un domaine très actif à l’interface des mathématiques et de l’in- formatique auquel ce travail se rattache. Les trois contributions de cette thèse concernent le développement et l’étude d’algorithmes topologiques pour calculer des décompositions et des déformations d’objets de basse dimension, comme des graphes, des surfaces ou des 3-variétés. -

The Word Problem for Braided Monoidal Categories Is Unknot-Hard
The word problem for braided monoidal categories is unknot-hard Antonin Delpeuch Jamie Vicary Department of Computer Science, University of Oxford Computer Laboratory, University of Cambridge [email protected] [email protected] We show that the word problem for braided monoidal categories is at least as hard as the unknotting problem. As a corollary, so is the word problem for tricategories. We conjecture that the word problem for tricategories is decidable. Introduction The word problem for an algebraic structure is the decision problem which consists in determining whether two expressions denote the same elements of such a structure. Depending on the equational theory of the structure, this problem can be very simple or extremely difficult, and studying it from the lens of complexity or computability theory has proved insightful in many cases. This work is the third episode of a series of articles studying the word problem for various sorts of categories, after monoidal categories [6] and double categories [5]. We turn here to braided monoidal categories. Unlike the previous episodes, we do not propose an algorithm deciding equality, but instead show that this word problem seems to be a difficult one. More precisely we show that it is at least as hard as the unknotting problem. The unknotting problem consists in determining whether a knot can be untied and was first formu- lated by Dehn in 1910 [3]. The decidability of this problem remained open until Haken gave the first algorithm for it in 1961 [9]. As of today, no polynomial time algorithm is known for it. -
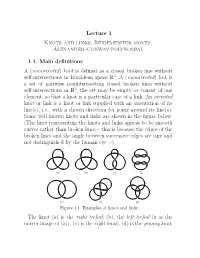
Lecture 1 Knots and Links, Reidemeister Moves, Alexander–Conway Polynomial
Lecture 1 Knots and links, Reidemeister moves, Alexander–Conway polynomial 1.1. Main definitions A(nonoriented) knot is defined as a closed broken line without self-intersections in Euclidean space R3.A(nonoriented) link is a set of pairwise nonintertsecting closed broken lines without self-intersections in R3; the set may be empty or consist of one element, so that a knot is a particular case of a link. An oriented knot or link is a knot or link supplied with an orientation of its line(s), i.e., with a chosen direction for going around its line(s). Some well known knots and links are shown in the figure below. (The lines representing the knots and links appear to be smooth curves rather than broken lines – this is because the edges of the broken lines and the angle between successive edges are tiny and not distinguished by the human eye :-). (a) (b) (c) (d) (e) (f) (g) Figure 1.1. Examples of knots and links The knot (a) is the right trefoil, (b), the left trefoil (it is the mirror image of (a)), (c) is the eight knot), (d) is the granny knot; 1 2 the link (e) is called the Hopf link, (f) is the Whitehead link, and (g) is known as the Borromeo rings. Two knots (or links) K, K0 are called equivalent) if there exists a finite sequence of ∆-moves taking K to K0, a ∆-move being one of the transformations shown in Figure 1.2; note that such a transformation may be performed only if triangle ABC does not intersect any other part of the line(s). -
![Arxiv:2104.14076V1 [Math.GT] 29 Apr 2021 Gauss Codes for Each of the Unknot Diagrams Discussed in This Note in Ap- Pendixa](https://docslib.b-cdn.net/cover/9961/arxiv-2104-14076v1-math-gt-29-apr-2021-gauss-codes-for-each-of-the-unknot-diagrams-discussed-in-this-note-in-ap-pendixa-2039961.webp)
Arxiv:2104.14076V1 [Math.GT] 29 Apr 2021 Gauss Codes for Each of the Unknot Diagrams Discussed in This Note in Ap- Pendixa
HARD DIAGRAMS OF THE UNKNOT BENJAMIN A. BURTON, HSIEN-CHIH CHANG, MAARTEN LÖFFLER, ARNAUD DE MESMAY, CLÉMENT MARIA, SAUL SCHLEIMER, ERIC SEDGWICK, AND JONATHAN SPREER Abstract. We present three “hard” diagrams of the unknot. They re- quire (at least) three extra crossings before they can be simplified to the 2 trivial unknot diagram via Reidemeister moves in S . Both examples are constructed by applying previously proposed methods. The proof of their hardness uses significant computational resources. We also de- termine that no small “standard” example of a hard unknot diagram 2 requires more than one extra crossing for Reidemeister moves in S . 1. Introduction Hard diagrams of the unknot are closely connected to the complexity of the unknot recognition problem. Indeed a natural approach to solve instances of the unknot recognition problem is to untangle a given unknot diagram by Reidemeister moves. Complicated diagrams may require an exhaustive search in the Reidemeister graph that very quickly becomes infeasible. As a result, such diagrams are the topic of numerous publications [5, 6, 11, 12, 15], as well as discussions by and resources provided by leading researchers in low-dimensional topology [1,3,7, 14]. However, most classical examples of hard diagrams of the unknot are, in fact, easy to handle in practice. Moreover, constructions of more difficult examples often do not come with a rigorous proof of their hardness [5, 12]. The purpose of this note is hence two-fold. Firstly, we provide three un- knot diagrams together with a proof that they are significantly more difficult to untangle than classical examples. -

Rectangular Knot Diagrams Classification with Deep Learning
Rectangular knot diagrams classification with deep learning L.H. Kauffman ∗† N.E. Russkikh ‡ I.A. Taimanov §¶‖ Abstract In this article we discuss applications of neural networks to recognising knots and, in particular, to the unknotting problem. One of motivations for this study is to understand how neural networks work on the example of a problem for which rigorous mathematical algorithms for its solution are known. We represent knots by rectangular Dynnikov diagrams and apply neural networks to distinguish a given diagram's class from the given finite families of topological types. The data presented to the program is generated by applying Dynnikov moves to initial samples. The significance of using these diagrams and moves is that in this context the problem of determining whether a diagram is unknotted is a finite search of a bounded combinatorial space. 1 Introduction In this article we discuss the applications of neural networks to recognising knots and, in particular, to the unknotting problem which appears to be the most interesting application. We represent knots by rectangular diagrams (see the text of the paper for the definition of these diagrams and for the moves that we use in transforming ∗Department of Mathematics, Statistics and Computer Science 851 South Morgan Street University of Illinois at Chicago Chicago, Illinois 60607-7045 and Department of Mechanics and Mathematics, Novosibirsk State University, Novosibirsk Russia †Kauffman’s work was supported by the Laboratory of Topology and Dynamics, Novosi- birsk State University (contract no. 14.Y26.31.0025 with the Ministry of Education and arXiv:2011.03498v1 [math.GT] 6 Nov 2020 Science of the Russian Federation). -

Algorithmic Simplification of Knot Diagrams
Algorithmic simplification of knot diagrams: new moves and experiments Carlo Petronio∗ Adolfo Zanellati August 21, 2018 Abstract This note has an experimental nature and contains no new theorems. We introduce certain moves for classical knot diagrams that for all the very many examples we have tested them on give a monotonic complete simplification. A complete simplification of a knot diagram D is a sequence of moves that transform D into a diagram D′ with the minimal possible number of crossings for the isotopy class of the knot represented by D. The simplification is monotonic if the number of crossings never increases along the sequence. Our moves are certain Z1,2,3 generalizing the classical Reidemeister moves R1,2,3, and another one C (together with a variant C) aimed at detecting whether a knot diagram can be viewed as a connectede sum of two easier ones. We present an accurate description of the moves and several results of our implementation of the simplification procedure based on them, publicly available on the web. MSC (2010): 57M25. Keywords: Knot diagram, move, simplification. This paper describes constructions and experimental results originally due arXiv:1508.03226v3 [math.GT] 6 Jun 2016 to the second named author, that were formalized, put into context, and generalized in collaboration with the first named author. No new theorem is proved. We introduce new combinatorial moves Z1,2,3 on knot diagrams, together with another move C and a variant C of C, that perform very well in the task of simplifying diagrams. Namely,e as we have checked by implementing the moves in [29] and applying them to a wealth of examples, the moves are ∗ Partially supported by the Italian FIRB project “Geometry and topology of low- dimensional manifolds” RBFR10GHHH. -

1 Basic Definitions
MA3F2 Definitions These notes for MA3F2 are an adaptation of Brian Sanderson’s notes, posted with his permission. The originals are available on the web at maths.warwick.ac.uk/ bjs/MA3F2-page.html. Any errors below are∼due to me; please inform me of such via email at [email protected]. 1 Basic definitions 1.1 Knots and their diagrams A knot K is a smooth loop in three-space which does not self-intersect itself. A more precise definition might read: Let S1 = (x, y) R2 x2 + y2 = 1 be the unit circle in the plane. A knot { ∈ | } K R3 is the image of a smooth embedding f : S1 R3. ⊂ → Since it is difficult to draw in R3, and easy to draw in the plane, we will visualize knots by projecting onto the xy–plane, and recording crossing information. So we define a knot diagram D to be a smooth loop in the plane which is allowed to transversely self-intersect at crossings. At each crossing there is exactly one overpassing and one underpassing arc. Here are a few examples: Figure 1: Diagrams of the unknot, the trefoil, and the figure eight. These are drawn as diagrams of polygonal knots. The unknot is special: it is the only knot which is not knotted. Here are a few drawings which are not diagrams of knots: Figure 2: Projections of an arc, a wild knot, a self-intersecting loop, and a loop with a triple point. We can generalize the notion of a knot to include links: a link L is a collection of pairwise disjoint knots in R3.