Plexopathies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dental Plexopathy Vesta Guzeviciene, Ricardas Kubilius, Gintautas Sabalys
SCIENTIFIC ARTICLES Stomatologija, Baltic Dental and Maxillofacial Journal, 5:44-47, 2003 Dental Plexopathy Vesta Guzeviciene, Ricardas Kubilius, Gintautas Sabalys SUMMARY Aim and purpose of the study were: 1) to study and compare unfavorable factors playing role in the development of upper teeth plexitis and upper teeth plexopathy; 2) to study peculiarities of clinical manifestation of upper teeth plexitis and upper teeth plexopathy, and to establish their diagnostic value; 3) to optimize the treatment. The results of examination and treatment of 79 patients with upper teeth plexitis (UTP-is) and 63 patients with upper teeth plexopathy (UTP-ty) are described in the article. Questions of the etiology, pathogenesis and differential diagnosis are discussed, methods of complex medicamental and surgical treatment are presented. Keywords: atypical facial neuralgia, atypical odontalgia, atypical facial pain, vascular toothache. PREFACE Besides the common clinical tests, in order to ana- lyze in detail the etiology and pathogenesis of the afore- Usually the injury of the trigeminal nerve is re- mentioned disease, its clinical manifestation and pecu- lated to the pathology of the teeth neural plexuses. liarities, we performed specific examinations such as According to the literature data, injury of the upper orthopantomography of the infraorbital canals, mea- teeth neural plexuses makes more than 7% of all sured the velocity of blood flow in the infraorbital blood neurostomatologic diseases. Many terms are used in vessels (doplerography), examined the pain threshold literature to characterize the clinical symptoms com- of facial skin and oral mucous membrane in acute pe- plex of the above-mentioned pathology. Some authors riod and remission, and evaluated the role that the state (1, 2, 3) named it dental plexalgia or dental plexitis. -

Peroneal Neuropathy Misdiagnosed As L5 Radiculopathy: a Case Report Michael D Reife1,2* and Christopher M Coulis3,4,5
Reife and Coulis Chiropractic & Manual Therapies 2013, 21:12 http://www.chiromt.com/content/21/1/12 CHIROPRACTIC & MANUAL THERAPIES CASE REPORT Open Access Peroneal neuropathy misdiagnosed as L5 radiculopathy: a case report Michael D Reife1,2* and Christopher M Coulis3,4,5 Abstract Objective: The purpose of this case report is to describe a patient who presented with a case of peroneal neuropathy that was originally diagnosed and treated as a L5 radiculopathy. Clinical features: A 53-year old female registered nurse presented to a private chiropractic practice with complaints of left lateral leg pain. Three months earlier she underwent elective left L5 decompression surgery without relief of symptoms. Intervention and outcome: Lumbar spine MRI seven months prior to lumbar decompression surgery revealed left neural foraminal stenosis at L5-S1. The patient symptoms resolved after she stopped crossing her legs. Conclusion: This report discusses a case of undiagnosed peroneal neuropathy that underwent lumbar decompression surgery for a L5 radiculopathy. This case study demonstrates the importance of a thorough clinical examination and decision making that ensures proper patient diagnosis and management. Keywords: Peroneal neuropathy, Lumbar radiculopathy, Chiropractic Background Case presentation The most common entrapment neuropathy in the lo- The patient is a 53-year-old registered nurse who was wer extremity is common peroneal mononeuropathy, ac- referred to the author’s office in August 2003 by her pri- counting for approximately 15% of all mononeuropathies mary care physician with a chief complaint of left leg in adults [1]. Most injuries occur at the fibular head and pain. Her symptoms began in October 2002 after she fell can be the result of many factors including chronic low off an ambulance landing on her left hip. -

Intrapartum Lesions to the Lumbar Portion of the Lumbosacral Plexus: an Anatomical Review
REVIEW Eur. J. Anat. 23 (2): 83-90 (2019) Intrapartum lesions to the lumbar portion of the lumbosacral plexus: an anatomical review Shanna E. Williams, Asa C. Black, Jr. Department of Biomedical Sciences, University of South Carolina School of Medicine Greenville, Greenville, SC, USA SUMMARY Key words: Plexopathy – Radiculopathy – Neu- The lumbosacral plexus is formed by the ventral ropathy – Pregnancy – Foot drop rami of L2-S3 and provides sensory and motor branches to the lower extremity. The spatial orien- INTRODUCTION tation of the lumbar portion of the plexus above the pelvic brim leaves it particularly susceptible to in- The lumbosacral plexus is formed by the ventral trapartum injury by the fetal head. Such lesions are rami of the L2-S3 segments, with some contribu- subdivided into two groups: upper lumbar plexus tions from L1 and S4 segments. It gives rise to six (L1-L4) and lumbosacral trunk (L4-L5). Given the sensory nerves of the thigh and leg, and six major root levels involved, upper lumbar plexus lesions sensorimotor nerves responsible for innervating 43 produce symptoms suggestive of iliohypogastric, muscles of the lower extremity (Van Alfen and ilioinguinal, genitofemoral, femoral, and obturator Malessy, 2013). As the name would suggest, it neuropathies or L4 radiculopathies. Alternatively, consists of two components, the lumbar plexus involvement of the lumbosacral trunk can imitate a and the sacral plexus, which are spatially separat- common fibular (peroneal) neuropathy or L5 ed. This anatomical separation results in a clinical radiculopathy. This symptomatic overlap with vari- division of lumbosacral plexus lesions into those ous neuropathies and radiculopathies, makes di- affecting the lumbar plexus and those affecting the agnosis of such lesions particularly challenging. -

Ischaemic Lumbosacral Plexopathy in Acute Vascular Compromise:Case Report
Parapkgia 29 (1991) 70-75 © 1991 International Medical Soci<ty of Paraplegia Paraplegia L-_________________________________________________ � Ischaemic Lumbosacral Plexopathy in Acute Vascular Compromise: Case Report D.X. Cifu, MD, K.D. Irani, MD Department of Physical Medicine, Baylor College of Medicine, Houston, Texas, USA. Summary Anterior spinal artery syndrome (ASAS) is a well reported cause of spinal cord injury (SCI) following thoracoabdominal aortic surgery. The resultant deficits are often incom plete, typically attributed to the variable nature of the vascular distribution. Our Physi cal Medicine and Rehabilitation (PM and Rehabilitation) service was consulted about a 36-year-old patient with generalised deconditioning, 3 months after a stab wound to the left ventricle. Physical examination revealed marked lower extremity weakness, hypo tonia, hyporeflexia, and a functioning bowel and bladder. Further questioning disclosed lower extremity dysesthesias. Nerve conduction studies showed slowed velocities, pro longed distal latencies and decreased amplitudes of all lower extremity nerves. Electro myography revealed denervation of all proximal and distal lower extremity musculature, with normal paraspinalis. Upper extremity studies were normal. Recently, 3 cases of ischaemic lumbosacral plexopathy, mimicking an incomplete SCI, have been reported. This distinction is particularly difficult in the poly trauma patient with multiple musculo skeletal injuries or prolonged recuperation time, in addition to a vascular insult, as in this patient. The involved anatomical considerations will be discussed. A review of the elec trodiagnostic data from 30 patients, with lower extremity weakness following acute ischaemia, revealed a 20% incidence of spinal cord compromise, but no evidence of a plexopathy. Key words: Ischaemia; Lumbosacral plexopathy; Electromyography. Recent advances in cardiovascular and trauma surgery have led to increased survi val of patients following cardiac and great vessel trauma or insult. -

Brachial-Plexopathy.Pdf
Brachial Plexopathy, an overview Learning Objectives: The brachial plexus is the network of nerves that originate from cervical and upper thoracic nerve roots and eventually terminate as the named nerves that innervate the muscles and skin of the arm. Brachial plexopathies are not common in most practices, but a detailed knowledge of this plexus is important for distinguishing between brachial plexopathies, radiculopathies and mononeuropathies. It is impossible to write a paper on brachial plexopathies without addressing cervical radiculopathies and root avulsions as well. In this paper will review brachial plexus anatomy, clinical features of brachial plexopathies, differential diagnosis, specific nerve conduction techniques, appropriate protocols and case studies. The reader will gain insight to this uncommon nerve problem as well as the importance of the nerve conduction studies used to confirm the diagnosis of plexopathies. Anatomy of the Brachial Plexus: To assess the brachial plexus by localizing the lesion at the correct level, as well as the severity of the injury requires knowledge of the anatomy. An injury involves any condition that impairs the function of the brachial plexus. The plexus is derived of five roots, three trunks, two divisions, three cords, and five branches/nerves. Spinal roots join to form the spinal nerve. There are dorsal and ventral roots that emerge and carry motor and sensory fibers. Motor (efferent) carries messages from the brain and spinal cord to the peripheral nerves. This Dorsal Root Sensory (afferent) carries messages from the peripheral to the Ganglion is why spinal cord or both. A small ganglion containing cell bodies of sensory NCS’s sensory fibers lies on each posterior root. -

Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions
Hindawi International Journal of Rheumatology Volume 2020, Article ID 2919625, 13 pages https://doi.org/10.1155/2020/2919625 Review Article Clinical Presentations of Lumbar Disc Degeneration and Lumbosacral Nerve Lesions Worku Abie Liyew Biomedical Science Department, School of Medicine, Debre Markos University, Debre Markos, Ethiopia Correspondence should be addressed to Worku Abie Liyew; [email protected] Received 25 April 2020; Revised 26 June 2020; Accepted 13 July 2020; Published 29 August 2020 Academic Editor: Bruce M. Rothschild Copyright © 2020 Worku Abie Liyew. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Lumbar disc degeneration is defined as the wear and tear of lumbar intervertebral disc, and it is mainly occurring at L3-L4 and L4-S1 vertebrae. Lumbar disc degeneration may lead to disc bulging, osteophytes, loss of disc space, and compression and irritation of the adjacent nerve root. Clinical presentations associated with lumbar disc degeneration and lumbosacral nerve lesion are discogenic pain, radical pain, muscular weakness, and cutaneous. Discogenic pain is usually felt in the lumbar region, or sometimes, it may feel in the buttocks, down to the upper thighs, and it is typically presented with sudden forced flexion and/or rotational moment. Radical pain, muscular weakness, and sensory defects associated with lumbosacral nerve lesions are distributed on -
4-Brachial Plexus and Lumbosacral Plexus (Edited).Pdf
Color Code Brachial Plexus and Lumbosacral Important Doctors Notes Plexus Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives At the end of this lecture, the students should be able to : Describe the formation of brachial plexus (site, roots) List the main branches of brachial plexus Describe the formation of lumbosacral plexus (site, roots) List the main branches of lumbosacral plexus Describe the important Applied Anatomy related to the brachial & lumbosacral plexuses. Brachial Plexus Formation Playlist o It is formed in the posterior triangle of the neck. o It is the union of the anterior rami (or ventral) of the 5th ,6th ,7th ,8th cervical and the 1st thoracic spinal nerves. o The plexus is divided into 5 stages: • Roots • Trunks • Divisions • Cords • Terminal branches Really Tired? Drink Coffee! Brachial Plexus A P A P P A Brachial Plexus Trunks Divisions Cords o Upper (superior) trunk o o Union of the roots of Each trunk divides into Posterior cord: C5 & C6 anterior and posterior From the 3 posterior division divisions of the 3 trunks o o Middle trunk Lateral cord: From the anterior Continuation of the divisions of the upper root of C7 Branches and middle trunks o All three cords will give o Medial cord: o Lower (inferior) trunk branches in the axilla, It is the continuation of Union of the roots of the anterior division of C8 & T1 those will supply their respective regions. the lower trunk The Brachial Plexus Long Thoracic (C5,6,7) Anterior divisions Nerve to Subclavius(C5,6) Posterior divisions Dorsal Scapular(C5) Suprascapular(C5,6) upper C5 trunk Lateral Cord C6 middle (2LM) trunk C7 lower C8 trunk T1 Posterior Cord (ULTRA) Medial Cord (4MU) In the PowerPoint presentation this slide is animated. -

Neuropathy, Radiculopathy & Myelopathy
Neuropathy, Radiculopathy & Myelopathy Jean D. Francois, MD Neurology & Neurophysiology Purpose and Objectives PURPOSE Avoid Confusing Certain Key Neurologic Concepts OBJECTIVES • Objective 1: Define & Identify certain types of Neuropathies • Objective 2: Define & Identify Radiculopathy & its causes • Objective 3: Define & Identify Myelopathy FINANCIAL NONE DISCLOSURE Basics What is Neuropathy? • The term 'neuropathy' is used to describe a problem with the nerves, usually the 'peripheral nerves' as opposed to the 'central nervous system' (the brain and spinal cord). It refers to Peripheral neuropathy • It covers a wide area and many nerves, but the problem it causes depends on the type of nerves that are affected: • Sensory nerves (the nerves that control sensation>skin) causing cause tingling, pain, numbness, or weakness in the feet and hands • Motor nerves (the nerves that allow power and movement>muscles) causing weakness in the feet and hands • Autonomic nerves (the nerves that control the systems of the body eg gut, bladder>internal organs) causing changes in the heart rate and blood pressure or sweating • It May produce Numbness, tingling,(loss of sensation) along with weakness. It can also cause pain. • It can affect a single nerve (mononeuropathy) or multiple nerves (polyneuropathy) Neuropathy • Symptoms usually start in the longest nerves in the body: Feet & later on the hands (“Stocking-glove” pattern) • Symptoms usually spread slowly and evenly up the legs and arms. Other body parts may also be affected. • Peripheral Neuropathy can affect people of any age. But mostly people over age 55 • CAUSES: Neuropathy has a variety of forms and causes. (an injury systemic illness, an infection, an inherited disorder) some of the causes are still unknown. -

Surgery for Lumbar Radiculopathy/ Sciatica Final Evidence Report
Surgery for Lumbar Radiculopathy/ Sciatica Final evidence report April 13, 2018 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box 42712 Olympia, WA 98504-2712 (360) 725-5126 www.hca.wa.gov/hta [email protected] Prepared by: RTI International–University of North Carolina Evidence-based Practice Center Research Triangle Park, NC 27709 www.rti.org This evidence report is based on research conducted by the RTI-UNC Evidence-based Practice Center through a contract between RTI International and the State of Washington Health Care Authority (HCA). The findings and conclusions in this document are those of the authors, who are responsible for its contents. The findings and conclusions do not represent the views of the Washington HCA and no statement in this report should be construed as an official position of Washington HCA. The information in this report is intended to help the State of Washington’s independent Health Technology Clinical Committee make well-informed coverage determinations. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information (i.e., in the context of available resources and circumstances presented by individual patients). This document is in the public domain and may be used and reprinted without permission except those copyrighted materials that are clearly noted in the document. Further reproduction of those copyrighted materials is prohibited without the specific permission of copyright holders. -

Lumbosacral Plexus Entrapment Syndrome. Part One: a Common Yet Little-Known Cause of Chronic Pelvic and Lower Extremity Pain
3-A Running head: ANAESTHESIA, PAIN & INTENSIVE CARE www.apicareonline.com ORIGINAL ARTICLE Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain Kjetil Larsen, CES, George C. Chang Chien, D O2 ABSTRACT Corrective exercise specialist, Training & Rehabilitation, Oslo Lumbosacral plexus entrapment syndrome (LPES) is a little-known but common cause Norway of chronic lumbopelvic and lower extremity pain. The lumbar plexus, including the 2 Director of pain management, lumbosacral tunks emerge through the fibers of the psoas major, and the proximal Ventura County Medical Center, sciatic nerve beneath the piriformis muscles. Severe weakness of these muscles may Ventura, CA 93003, USA. lead to entrapment plexopathy, resulting in diffuse and non-specific pain patterns Correspondence: Kjetil Larsen, CES, Corrective throughout the lumbopelvic complex and lower extremities (LPLE), easily mimicking Exercise Specialist, Training & other diagnoses and is therefore likely to mislead the interpreting clinician. It is a Rehabilitation, Oslo Norway; pathology very similar to that of thoracic outlet syndrome, but for the lower body. This Kjetil@trainingandrehabilitation. two part manuscript series was written in an attempt to demonstrate the existence, com; pathophysiology, diagnostic protocol as well as interventional strategy for LPES, and Tel.: +47 975 45 192 its efficacy. Received: 23 November 2018, Reviewed & Accepted: 28 Key words: Pelvic girdle; Pain, Pelvic girdle; Lumbosacral plexus entrapment syndrome; February 2019 Piriformis syndrome; Nerve entrapment; Double-crush; Pain, Chronic; Fibromyalgia Citation: Larsen K, Chien GCC. Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain. -

Patient Information
PATIENT INFORMATION Cervical Radiculopathy A MaineHealth Member What is a Cervical Radiculopathy? Cervical radiculopathy (ra·dic·u·lop·a·thy) is when a nerve in your neck gets irritated. It can cause pain numbness, tingling, or weakness. Neck pain does not mean you have a pinched nerve, although it may be present. What causes cervical radiculopathy? Factors that cause cervical radiculopathy include: ■■ Bulging or herniated discs ■■ Bone spurs These are all common and result from normal wear and tear. A nerve may be irritated by a particular activity (reaching, lifting), a trauma (such as a car accident or fall), or no clear cause at all, other than normal life activity. Smoking does increase the wear and tear so it is important to quit smoking. What is a herniated disc? Your discs act like cushions between the bones in the neck. When the outer coating of the disc (the annulus) weakens or is injured, it may no longer be able to protect the soft spongy material (the nucleus) in the middle of the disc. At first the disc may bulge, eventually the nucleus can break through the annulus. This is called a herniated disc. What is a bone spur? Bone spurs are caused by pressure and extra stress on the bones of the spine (or vertebrae). The body responds to this constant stress by adding extra bone, which results in a bone spur. Bone spurs can pinch or put pressure on a nerve. Maine Medical Center Neuroscience Institute Page 1 of 4 Cervical Radiculopathy What are the common signs and symptoms of cervical radiculopathy? ■■ Pain in neck, shoulder blades, shoulder, and/or arms ■■ Tingling or numbness in arms ■■ Weakness in arm muscles Limited functional ability for tasks as reaching, lifting and gripping, or prolonged head postures as in reading What is the treatment for cervical radiculopathy? Most spine problems heal over time without surgery within 6 to 12 weeks. -
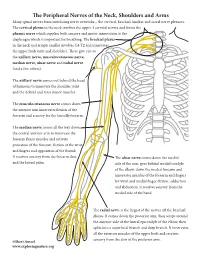
The Peripheral Nerves of the Neck, Shoulders and Arms Many Spinal Nerves Form Interlacing Nerve Networks – the Cervical, Brachial, Lumbar and Sacral Nerve Plexuses
The Peripheral Nerves of the Neck, Shoulders and Arms Many spinal nerves form interlacing nerve networks – the cervical, brachial, lumbar and sacral nerve plexuses. The cervical plexus in the neck involves the upper 4 cervical nerves and forms the phrenic nerve which supplies both sensory and motor innervation to the diaphragm which is important for breathing. The brachial plexus in the neck and armpit (axilla) involves C4-T2 and innervates the upper limb (arm and shoulder). These give rise to the axillary nerve, musculocutaneous nerve, median nerve, ulnar nerve and radial nerve (and a few others). The axillary nerve comes out behind the head of humerus to innervate the shoulder joint and the deltoid and teres minor muscles. The musculocutaneous nerve comes down the anterior arm innervates flexion of the forearm and sensory for the laterally forearm. The median nerve comes all the way down the central anterior arm to innervate the forearm flexor muscles and activate pronation of the forearm, flexion of the wrist and fingers and opposition of the thumb. It receives sensory from the forearm skin The ulnar nerve comes down the medial and the lateral palm. side of the arm, goes behind medial condyle of the elbow, down the medial forearm and innervates muscles of the forearm and fingers for wrist and medial finger flexion, adduction and abduction. It receives sensory from the medial side of the hand. The radial nerve is the largest of the nerves off the brachial plexus. It comes down the posterior arm, then wraps around the anterior aide of the lateral epicondyle of the elbow, then splits into a superficial branch and deep branch.