Akathisia As an Extrapyramidal Side Effect of Fluoxetine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Canadian Stroke Best Practice Recommendations
CANADIAN STROKE BEST PRACTICE RECOMMENDATIONS MOOD, COGNITION AND FATIGUE FOLLOWING STROKE Table 1C: Summary Table for Selected Pharmacotherapy for Post-Stroke Depression Update 2019 Lanctôt KL, Swartz RH (Writing Group Chairs) on Behalf of the Canadian Stroke Best Practice Recommendations Mood, Cognition and Fatigue following Stroke Writing Group and the Canadian Stroke Best Practice and Quality Advisory Committee, in collaboration with the Canadian Stroke Consortium © 2019 Heart & Stroke Heart and Stroke Foundation Mood, Cognition and Fatigue following Stroke Canadian Stroke Best Practice Recommendations Table 1C Table 1C: Summary Table for Selected Pharmacotherapy for Post-Stroke Depression This table provides a summary of the pharmacotherapeutic properties, side effects, drug interactions and other important information on selected classes of medications available for use in Canada and more commonly recommended for post-stroke depression. This table should be used as a reference guide by health care professionals when selecting an appropriate agent for individual patients. Patient compliance, patient preference and/or past experience, side effects, and drug interactions should all be taken into consideration during decision-making, in addition to other information provided in this table and available elsewhere regarding these medications. Selective Serotonin Reuptake Inhibitors (SSRI) Serotonin–norepinephrine reuptake Other inhibitors (SNRI) Medication *citalopram – Celexa *duloxetine – Cymbalta methylphenidate – Ritalin (amphetamine) -

Free PDF Download
European Review for Medical and Pharmacological Sciences 2021; 25: 4746-4756 Pathophysiology and management of Akathisia 70 years after the introduction of the chlorpromazine, the first antipsychotic N. ZAREIFOPOULOS1, M. KATSARAKI1, P. STRATOS1, V. VILLIOTOU, M. SKALTSA1, A. DIMITRIOU1, M. KARVELI1, P. EFTHIMIOU2, M. LAGADINOU2, D. VELISSARIS3 1Department of Psychiatry, General Hospital of Nikea and Pireus Hagios Panteleimon, Athens, Greece 2Emergency Department, University General Hospital of Patras, Athens, Greece 3Department of Internal Medicine, University of Patras School of Medicine, Athens, Greece Abstract. – OBJECTIVE: Akathisia is among CONCLUSIONS: Pharmacological manage- the most troubling effects of psychiatric drugs ment may pose a challenge in chronic akathi- as it is associated with significant distress on sia. Rotation between different pharmacologi- behalf of the patients, and it limits treatment ad- cal management strategies may be optimal in re- herence. Though it most commonly presents sistant cases. Discontinuation of the causative during treatment with antipsychotic drugs which drug and use of b-blockers, mirtazapine, benzo- block dopamine D2 receptors, Akathisia has al- diazepines or gabapentinoids for symptomatic so been reported during treatment with selec- relief is the basis of management. tive serotonin reuptake inhibitors (SSRIs), se- rotonin norepinephrine reuptake inhibitors (SN- Key Words: RIs), stimulants, mirtazapine, tetrabenazine and Aripiprazole, Extrapyramidal symptoms, Haloperi- other drugs. dol, -

The Pharma-Fever That Almost Got Away
EMERGENCY MEDICINE RESIDENCY CPC The pharma-fever that almost got away XIAO CHI ZHANG, MD, MS; MATTHEW SIKET, MD; WILLIAM BINDER, MD 29 31 EN From the Case Records of the Alpert Medical School of DR. SARAH GAINES: A fever greater than 41.0°C is quite Brown University Residency in Emergency Medicine elevated and unusual. Is this dangerous? What was your differential? DR. XIAO CHI ZHANG: A 68-year-old man was brought into DR. MATTHEW SIKET: Humans generally tolerate tempera- the Emergency Department by his family with chills and tures below 41° C (105.8° F). In contrast to hyperthermia, in altered mental status. Two days prior to his ED presenta- which an imbalance between heat generation versus dissipa- tion, the patient had an episode in which he “spaced-out” tion occurs without up-regulation of the hypothalamic set and was unable to comprehend or acknowledge his wife. She point, fever as a host defense against infection rarely reaches reported that he did not have any signs of seizure activity dangerous levels in neurologically competent individuals. and did not have any focal weakness. The episode lasted Very high temperatures can be related to urosepsis, intraab- approximately 30 minutes and he returned to his baseline. dominal sepsis, C. difficile colitis, meningitis, and central Today he had another episode, but this time associated with venous catheter infections. Hyperpyrexia, defined as tem- chills and rigors. His past medical history was significant perature > 41.5°C (106.7°F) is an uncommon result of infec- for chronic back pain due to bony metastasis from Stage IV tion and usually implies central fever, neurologic malignant non-small cell lung adenocarcinoma, requiring palliative syndrome, malignant hyperthermia, adrenal insufficiency, gamma knife radiation, as well as a daily oral chemotherapy or a drug related cause.1 Our patient was hyperpyrexic, sug- agent, erlotinib, an oral tyrosine kinase inhibitor. -

Efficacy of Antimanic Treatments: Meta-Analysis of Randomized, Controlled Trials
Neuropsychopharmacology (2011) 36, 375–389 & 2011 American College of Neuropsychopharmacology. All rights reserved 0893-133X/11 $32.00 www.neuropsychopharmacology.org Efficacy of Antimanic Treatments: Meta-analysis of Randomized, Controlled Trials ,1,2 2,3 4 2 Ays¸egu¨l Yildiz* , Eduard Vieta , Stefan Leucht and Ross J Baldessarini 1 2 Department of Psychiatry, Dokuz Eylu¨l University, Izmir, Turkey; Department of Psychiatry, Harvard Medical School and International Consortium for Bipolar Disorder Research and Psychopharmacology Program, McLean Division of Massachusetts General Hospital, Boston, 3 Massachusetts; Bipolar Disorders Program, Institute of Clinical Neuroscience, Hospital Clinic, University of Barcelona, IDIBAPS, CIBERSAM, 4 Barcelona, Spain; Department of Psychiatry and Psychotherapy, Klinik fu¨r Psychiatrie und Psychotherapie der TU-Mu¨nchen, Klinikum rechts der Isar, Technische Universita¨t Mu¨nchen, Mu¨nchen, Germany We conducted meta-analyses of findings from randomized, placebo-controlled, short-term trials for acute mania in manic or mixed states of DSM (III–IV) bipolar I disorder in 56 drug–placebo comparisons of 17 agents from 38 studies involving 10 800 patients. Of drugs tested, 13 (76%) were more effective than placebo: aripiprazole, asenapine, carbamazepine, cariprazine, haloperidol, lithium, olanzapine, paliperdone, quetiapine, risperidone, tamoxifen, valproate, and ziprasidone. Their pooled effect size for mania improvement (Hedges’ g in 48 trials) was 0.42 (confidence interval (CI): 0.36–0.48); pooled responder risk ratio (46 trials) was 1.52 (CI: 1.42–1.62); responder rate difference (RD) was 17% (drug: 48%, placebo: 31%), yielding an estimated number-needed-to-treat of 6 (all po0.0001). In several direct comparisons, responses to various antipsychotics were somewhat greater or more rapid than lithium, valproate, or carbamazepine; lithium did not differ from valproate, nor did second generation antipsychotics differ from haloperidol. -

PRESCRIBED DRUGS and NEUROLOGICAL COMPLICATIONS K a Grosset, D G Grosset Iii2
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2004.045757 on 16 August 2004. Downloaded from PRESCRIBED DRUGS AND NEUROLOGICAL COMPLICATIONS K A Grosset, D G Grosset iii2 J Neurol Neurosurg Psychiatry 2004;75(Suppl III):iii2–iii8. doi: 10.1136/jnnp.2004.045757 treatment history is a fundamental part of the healthcare consultation. Current drugs (prescribed, over the counter, herbal remedies, drugs of misuse) and how they are taken A(frequency, timing, missed and extra doses), drugs tried previously and reason for discontinuation, treatment response, adverse effects, allergies, and intolerances should be taken into account. Recent immunisations may also be of importance. This article examines the particular relevance of medication in patients presenting with neurological symptoms. Drugs and their interactions may contribute in part or fully to the neurological syndrome, and treatment response may assist diagnostically or in future management plans. Knowledge of medicine taking behaviour may clarify clinical presentations such as analgesic overuse causing chronic daily headache, or severe dyskinesia resulting from obsessive use of dopamine replacement treatment. In most cases, iatrogenic symptoms are best managed by withdrawal of the offending drug. Indirect mechanisms whereby drugs could cause neurological problems are beyond the scope of the current article—for example, drugs which raise blood pressure or which worsen glycaemic control and consequently increase the risk of cerebrovascular disease, or immunosupressants -

Cariprazine Exhibits Anxiolytic and Dopamine D Receptor-Dependent
International Journal of Neuropsychopharmacology (2017) 20(10): 788–796 doi:10.1093/ijnp/pyx038 Advance Access Publication: May 22, 2017 Regular Research Article regular research article Cariprazine Exhibits Anxiolytic and Dopamine D3 Receptor-Dependent Antidepressant Effects in the Chronic Stress Model Vanja Duric, Mounira Banasr, Tina Franklin, Ashley Lepack, Nika Adham, Béla Kiss, István Gyertyán, Ronald S. Duman Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut (Dr Duric, Dr Banasr, Dr Franklin, Ms Lepack, and Dr Duman); Department of Physiology and Pharmacology, Des Moines University, Des Moines, Iowa (Dr Duric); Campell Family Mental Health Research Institute of CAMH, Toronto, Ontario, Canada (Dr Banasr); Department of Pharmacology, Allergan, Jersey City, New Jersey (Dr Adham); Pharmacological and Safety Research, Gedeon Richter Plc, Budapest, Hungary (Dr Kiss); MTA-SE NAP B Cognitive Translational Behavioral Pharmacology Group, Department of Pharmacology and Pharmacotherapy, Semmelweis University, Budapest, Hungary (Dr Gyertyán); Institute of Cognitive Neuroscience and Psychology, Hungarian Academy of Sciences, Budapest, Hungary (Dr Gyertyán). Correspondence: Ronald S. Duman, PhD, Professor of Psychiatry and Pharmacology, Director, Abraham Ribicoff Research Facilities, Laboratory of Molecular Psychiatry, Yale University School of Medicine, 34 Park Street, Room 308, New Haven, CT 06508 ([email protected]). Abstract Background: Cariprazine, a D3-preferring dopamine D2/D3 receptor partial agonist, is a new antipsychotic drug recently approved in the United States for the treatment of schizophrenia and bipolar mania. We recently demonstrated that cariprazine also has significant antianhedonic-like effects in rats subjected to chronic stress; however, the exact mechanism of action for cariprazine’s antidepressant-like properties is not known. -
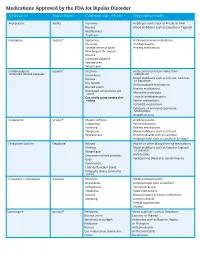
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common Side Effects May Interact With
Medications Approved by the FDA for Bipolar Disorder Medication Brand Name Common side effects May interact with Aripiprazole Abilify® Insomnia Antidepressants such as Prozac or Paxil Nausea Mood stabilizers such as Equetro or Tegretol Restlessness Tiredness Asenapine Saphris® Sleepiness Antihypertensive medications Dizziness Antidepressants Strange sense of taste Anxiety medications Numbing of the mouth Nausea Increased appetite Feeling tired Weight gain Carbamazepine Equetro™ Dizziness Birth control pills (can make them extended release capsules Drowsiness ineffective) Mood stabilizers such as Lithium, Lamictal, Nausea or Depakote Dry mouth Anticonvulsant medications Blurred vision Anxiety medications Decreased white blood cell count Macrolide antibiotics Can rarely cause severe skin Tricyclic antidepressants rashes Cancer medications HIV/AIDS medications Cytotoxic or immunosuppressive Medications Grapefruit juice Cariprazine Vraylar® Muscle stiffness Antidepressants Indigestion Pain medications Vomiting Anxiety medications Sleepiness Mood stabilizers such as Lithium Restlessness Anticonvulsants such as Lamictal Antipsychotics such as Latuda or Seroquel Divalproex Sodium Depakote® Nausea Aspirin or other blood thinning medications Shaking Mood stabilizers such as Equetro,Tegretol, Weight gain or Lamictal Decrease in blood platelets Barbiturates Rash Cyclosporine (Neoral or Sandimmune) Pancreatitis Liver dysfunction (rare) Polycystic Ovary Syndrome (rare) Fluoxetine + Olanzapine Symbyax® Dizziness MAOI antidepressants Drowsiness Antipsychotics -

Managing Migraine
NEUROLOGY/EXPERT CLINICAL MANAGEMENT Managing Migraine Benjamin W. Friedman, MD, MS* *Corresponding Author. E-mail: [email protected], Twitter: @benjaminbwf. 0196-0644/$-see front matter Copyright © 2016 by the American College of Emergency Physicians. http://dx.doi.org/10.1016/j.annemergmed.2016.06.023 A podcast for this article is available at www.annemergmed.com. alertness, and appetite. Allodynia, an alteration of Continuing Medical Education exam for this article is available at nociception that causes typically non-noxious sensory http://www.acep.org/ACEPeCME/. stimuli (such as brushing one’s hair or shaving one’s face) to be perceived as painful, develops as acute migraine duration [Ann Emerg Med. 2017;69:202-207.] increases. This is thought to indicate involvement of higher-order central nervous system sensory relay stations, Editor’s Note: The Expert Clinical Management series notably, the thalamus. consists of shorter, practical review articles focused on the optimal approach to a specific sign, symptom, disease, Migraine was once believed to be a vascular headache. procedure, technology, or other emergency department Advanced imaging studies do not support this description challenge. These articles–typically solicited from and indicate that migraine is a neurologic disorder involving recognized experts in the subject area–will summarize the dysfunctional nociceptive processing.3 Abnormally activated best available evidence relating to the topic while including sensory pathways turn non-noxious stimuli into headache, practical recommendations where the evidence is photophobia, phonophobia, and osmophobia. Cortical incomplete or conflicting. spreading depression, a slow wave of brain depolarization, underlies migraine aura but has not been demonstrated clearly in migraine patients without aura. -

Catatonia, NMS, and Serotonin Syndrome
Catatonia, NMS, and Serotonin Syndrome Christopher M. Celano, MD, FACLP Associate Director, Cardiac Psychiatry Research Program, Massachusetts General Hospital Assistant Professor of Psychiatry, Harvard Medical School October 22, 2020 www.mghcme.org Disclosure: Christopher Celano, MD Sunovion Company Pharmaceuticals Employment Management Independent Contractor Consulting Speaking & Teaching I Board, Panel or Committee Membership D – Relationship is considered directly relevant to the presentation I – Relationship is NOT considered directly relevant to the presentation www.mghcme.org Catatonia: How common is it? • 7.8-9.0% prevalence rate – Highest rates in non-psychiatric (i.e., medical) settings and in patients undergoing ECT. • 1.6-5.5% of all patients seen on psychiatry consultation service – Prevalence higher for older patients • Up to 46% of cases may have etiology that is not primarily psychiatric Grover 2015, Carroll 1994, Jaimes-Albornoz 2013, Fricchione 2008 www.mghcme.org When are you called? • Staff reports the patient is “Playing POSSUM” • Perseveration (speech or behavior) • Oppositionality to all requests • Speech that trails off or is whispereD • Slowed response to questions or commands • Undernourished (reports of decreased PO intake) • Motionless but awake www.mghcme.org Diagnosing Catatonia: DSM-5 DSM-5 requires 3 or more of the following: • Catalepsy • Posturing • Waxy flexibility • Mannerisms • Stupor • Stereotypies • Agitation • Grimacing • Mutism • Echolalia • Negativism • Echopraxia American Psychiatric Association 2013 www.mghcme.org Bush-Francis Rating Scale • Excitement • VerBigeration • Immobility/stupor • Rigidity • ComBativeness • Negativism • Autonomic Abnormality • Waxy flexiBility • Impulsivity • Withdrawal • Mutism • Automatic OBedience • Staring • Mitgehen • Posturing/catalepsy • Gegenhalten • Grimacing • AmBitendency • Echopraxia/echolalia • Grasp Reflex • Stereotypy • Perseveration • Mannerisms Bush 1996 www.mghcme.org Challenges with Diagnosis • Clarifying specific symptoms can be difficult – Rigidity vs. -

Triptan Therapy for Acute Migraine
Triptan Therapy for Acute Migraine Headache John Farr Rothrock, MD University of Alabama at Birmingham, Birmingham, AL Deborah I. Friedman, MD, MPH University of Texas Southwestern, Dallas, TX The “triptans” are 5HT-1B/1D receptor agonists that were developed to treat acute migraine and acute cluster headache. Sumatriptan, the original triptan preparation, has been in general use since 1993, so there has been considerable experience with triptans over time. There are currently seven oral triptans on the market in the United States: sumatriptan (Imitrex™), naratriptan (Amerge™), zolmitriptan (Zomig™), rizatriptan (Maxalt™), almotriptan (Axert™), frovatriptan (Frova™), and eletriptan (Relpax™); brand names may differ by country. There is also a combination preparation of oral sumatriptan/naproxen (Treximet™). Two triptans (sumatriptan, zolmitriptan) are marketed as nasal sprays, and sumatriptan is available for subcutaneous injection, including a needle-free subcutaneous delivery system (Sumavel™). Sumatriptan suppositories are marketed in Europe but not in North America. Zolmitriptan and rizatriptan are sold in an oral disintegrating tablet or “melt” formulation as well as in tablet form; while the “melt” formulations may be more convenient (no liquid is required to propel them into the stomach), they are absorbed similarly to regular tablets and there is no evidence to suggest that they work faster than the tablet formulations. Some patients with migraine-associated nausea prefer the disintegrating tablets while others cannot tolerate their taste. Although all of the triptans initially were investigated for the treatment of migraine headache of moderate to severe intensity and were superior to placebo in those pivotal trials, they appear to be more consistently effective when used to treat migraine earlier in the attack, when the headache is still mild to moderate. -

Aggression and Agitation in Dementia, None of Which Are Approved by the US Food and Drug Administration
REVIEW ARTICLE 07/09/2018 on SruuCyaLiGD/095xRqJ2PzgDYuM98ZB494KP9rwScvIkQrYai2aioRZDTyulujJ/fqPksscQKqke3QAnIva1ZqwEKekuwNqyUWcnSLnClNQLfnPrUdnEcDXOJLeG3sr/HuiNevTSNcdMFp1i4FoTX9EXYGXm/fCfl4vTgtAk5QA/xTymSTD9kwHmmkNHlYfO by https://journals.lww.com/continuum from Downloaded Aggression and Agitation CONTINUUM AUDIO Downloaded INTERVIEW AVAILABLE in Dementia ONLINE from By M. Uri Wolf, MD, FRCPC; Yael Goldberg, PhD, CPsych; https://journals.lww.com/continuum Morris Freedman, MD, FRCPC, FAAN CITE AS: CONTINUUM (MINNEAP MINN) 2018;24(3,BEHAVIORALNEUROLOGY AND PSYCHIATRY):783–803. ABSTRACT Address correspondence to by Dr M. Uri Wolf, Baycrest Health SruuCyaLiGD/095xRqJ2PzgDYuM98ZB494KP9rwScvIkQrYai2aioRZDTyulujJ/fqPksscQKqke3QAnIva1ZqwEKekuwNqyUWcnSLnClNQLfnPrUdnEcDXOJLeG3sr/HuiNevTSNcdMFp1i4FoTX9EXYGXm/fCfl4vTgtAk5QA/xTymSTD9kwHmmkNHlYfO PURPOSEOFREVIEW: This article reviews the treatment of aggression and Sciences, 3560 Bathurst St, agitation in dementia. Both nonpharmacologic and pharmacologic Toronto, ON M6A 2E1, Canada, approaches to responsive behaviors are discussed. Practical treatment [email protected]. strategies are applied to common behavioral symptoms. RELATIONSHIP DISCLOSURE: Drs Wolf and Goldberg report no disclosures. Dr Freedman serves RECENT FINDINGS: Aggressive and agitated behavior is common in dementia. as a trustee for the World Behavioral symptoms lead to reduced quality of life and distress for both Federation of Neurology and on patients and caregivers. They can also lead to poor outcomes and are the editorial boards -

Neuroleptic Malignant Syndrome Vs Serotonin Syndrome: Can They Be Distinguished Without an Underlying Etiology?
Neuroleptic Malignant Syndrome vs Serotonin Syndrome: Can They Be Distinguished Without an Underlying Etiology? Roy R. Reeves, DO, PhD; Mark E. Ladner, MD; and Percy Smith, PA The potentially serious complications for patients with neuroleptic malignant syndrome and serotonin syndrome cannot be underplayed by mental health clinicians, patients, and their families. The authors discuss clinical similarities and diagnostic and treatment approaches to the 2 syndromes. euroleptic malignant syn- of symptoms or whether the patient ployment. He had stopped taking his drome (NMS) and sero- was taking different medications that medication (risperidone 3-mg daily) tonin syndrome (SS) are rare could affect both the serotonin and 3 months earlier, because he was Nbut potentially fatal condi- dopamine systems. experiencing adverse effects (AEs) tions associated with the treatment In clinical practice many patients and shortly thereafter hearing voices. of psychotropic medications. Neu- are treated concurrently with both The clinic psychiatrist started him on roleptic malignant syndrome is be- dopamine receptor antagonists and olanzapine 10-mg daily and sertra- lieved to be caused by a reduction in agents that increase serotonin activity. line 50-mg daily. At a follow-up visit dopaminergic activity secondary to Thus, the distinction of NMS and SS a week later, Mr. A showed an im- drug-induced dopaminergic block- may be problematic if these patients provement of his mood and reported age, whereas SS results from an ex- develop symptoms that could be at- that his hallucinations had decreased cess of central nervous system (CNS) tributed to either disorder. This article significantly. serotonin activity, usually because will discuss the clinical similarities of Several days later, Mr.